Learning the basic chemistry concepts, in which an entire chemical education process is based on, can be overwhelming for beginners.
One of the reasons is the vast amount of information that there is out there.
For that reason, I decided to go ahead and explain here 15 important basic chemistry concepts, which hopefully will get you in a better shape for learning chemistry. These are clearly explained in most of the chemistry textbooks in our review.
You might also be interest in taking a look to some basic organic chemistry books. Or maybe you are looking to prepare your AP chemistry exam, or the chemistry SAT tests!
In case you are starting to learn organic chemistry, we have also published an overview of the most important concepts that you will need, and a further review comparing SN1 and SN2 reactions.
We will start off with an introduction to basic chemistry: background on the most basic definitions of chemistry, a bit of history, and highlighting why and how chemistry is important. The basic units in chemistry will be defined: atoms, molecules, subatomic particles. Then, we will discuss them from a beginner point of view, and formulate them in the format of questions.
Therefore, we aim this article to people that are unfamiliar with chemistry or with science in general. If you are a teacher, you might want to redirect your students here.
Disclaimer: This is not intended to be a comprehensive description of each concept, but rather an introduction to each of them: chemistry basics for beginners. We cite and include sources that we consider useful for expanding them further.
So without further ado, lets dive in!
You can use the next table of contents to navigate directly to the basic chemistry concepts you are interested on the most:
1. What does chemistry mean?
As an introduction to chemistry, it is the branch of science that studies matter and change. First, chemistry deals with the study of the composition and the properties of matter (which is basically any macroscopic substance that we can observe). Then, chemistry deals with change, or how these substances evolve when submitted to certain conditions, or how one substance changes or reacts while interacting with a different substance. The definition of chemistry can’t be made shorter, since it covers basically everything!
2. When was chemistry “discovered”?
Very simple chemical processes were performed even during ancient history, at 1000 BC, much before any basic chemistry concepts or laws were actually established. Extraction of metals from ore or getting compounds out of natural sources such as plants, are examples of chemistry that was first performed thousands of years ago and are still a thing today.
Alchemy is what we call the “protoscience” of chemistry.

Alchemy tried to explain the nature, properties and transformations of matter. But it was not science, but rather a set of myths and magic. It is agreed upon that, the transition from alchemy to modern chemistry as we know it, started on the 17th century, with the publication of The Sceptical Chymist by Robert Boyle (1661), who is considered the father of modern chemistry. The difference between chemistry and alchemy is the application of the scientific method.
3. Why is chemistry important?
Everything is chemistry. Everything that you can observe macroscopically is made of chemicals. You are made of chemicals, your food is chemicals, you breathe chemicals, we live out of and thanks to chemicals, everything you see under the sun is a mixture of chemicals.
Everything you see or do is based on chemical concepts and processes. Fireworks going off takes place thanks to our understanding and application of chemistry. A medicine that you take to cure your illness does it so through chemical processes. A building doesn’t fall off because we know chemistry. Obviously, all these examples result from a bunch of different branches of science coming together. Chemistry, as the central science, is in charge of gluing them together.
4. Why is chemistry called central science?
A all sciences are glued together by basic chemistry concepts, thats why it is called the central science. Without chemistry, physical sciences (which include chemistry itself), would find a gap and not be bound to life sciences (such as biology) and applied sciences (such as engineering). This is important to note in any introduction to chemistry text.
5. What is the future of chemistry?
Chemistry is the science that studies and manipulates matter. We human beings are getting pretty good at it, (although it is not easy to beat Nature), but we are far from an ideal position in which we can easily make any molecule or compound at will in a matter of minutes.
That is probably the future of chemical synthesis, being able to shape any compound at will in a matter of minutes, without relying on long term challenging synthesis projects. Furthermore, the possibilities of synthetic chemistry are literally endless: there will always be room for making a chemical even better, or finding a molecule that works even better for a given task.
Another key aspect of the “chemistry of the future” will be reaching true full sustainability. Chemistry will be one of the main branches of science to solve the problem of energy.
Also, chemistry, as the central science, will be responsible to helping technology and interdisciplinary science in general to develop smoothly.
6. What is an atom?
An atom is defined as the basic unit of a chemical element. A fundamental piece of matter. Plain simple and basic chemistry concept.
Obviously we now know that atoms are made of smaller particles, called subatomic particles: protons (positively charged by convention), electrons (negatively charged by convention) and neutrons (neutral particles).
In simple terms: Protons and neutrons make up the inner part of the atom, or nucleus, and account for most of the mass of the atoms. Electrons make up a cloud (orbitals, or areas in which the probability of finding an electron is high) around the nucleus.
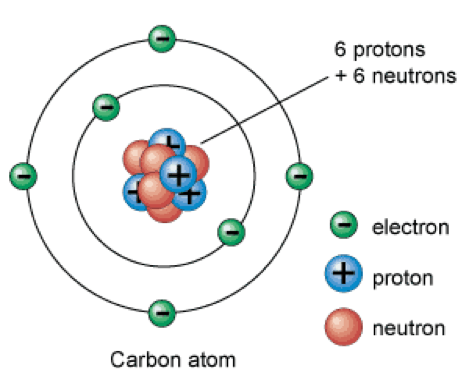
Protons and neutrons are not fundamental particles; they are made of quarks. On the other hands, as far as we known, electrons are fundamental particles and they are not made of anything smaller.
7. What is a molecule?
A molecule is a group of atoms bound together. It’s the next level of chemical complexity. Molecules represent the basic unit of a chemical compound, and this another of those essential basic chemistry concepts.
That’s basically it. An example is a water molecule, which is made from two atoms of hydrogen (H) and one atom of oxygen (O), held together via covalent bonds.
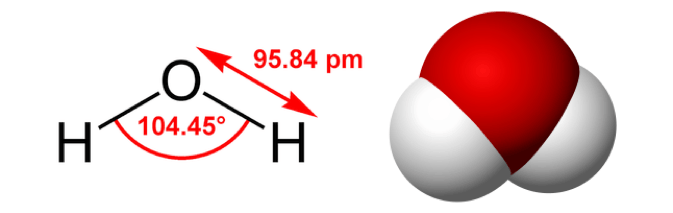
Further definitions include, that they are the simplest fundamental unit that can take part in a chemical reaction, and that they are electrically neutral. This latter point distinguishes molecules from ions, another type of chemical compound.
8. What types of chemical compounds are there?
There are three basic types of chemical compounds, and should be briefly introduced in this post about basic chemistry concepts. All of them are the result of bonding atoms together. The difference is in the nature of the forces that hold together those atoms.
- In molecules (such as water, or H2O, see above), which are neutral compounds of “individual” nature, atoms are glued together by covalent bonds. Covalent bonds generally occur between two non-metal atoms, which share pairs of electrons, or bonding pairs.
- In ionic compounds (such as sodium chloride, or NaCl, commonly known as “salts”), atoms are in ionic form (charged) and are held together by ionic forces, giving rise to large networks of oppositely charged ions. Ionic bonds occur between metals and non-metals.
- When extended networks of atoms are formed between one or more types of metal atoms, we are talking about metallic bonds.
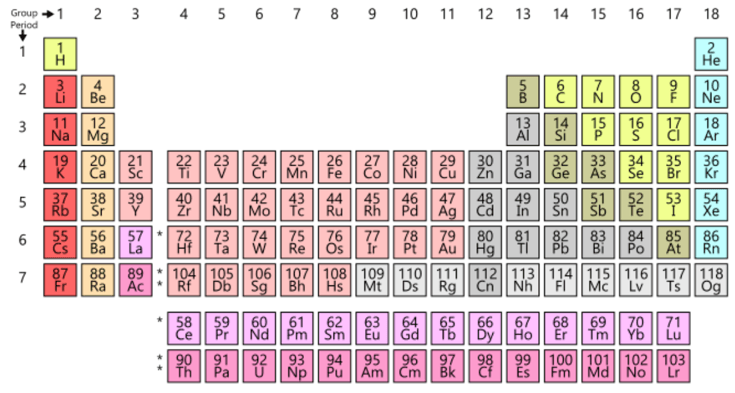
9. What is the periodic table?
The periodic table is a list or arrangement of all known chemical elements. These are organized in a way that it allows grouping elements with similar atomic structure, and therefore, similar properties. The main criteria for this order is the arrangement by increasing atomic number, which is the number of particles of that element’s nucleus (protons and neutrons). It’s invention is attributed to the Russian chemist Dimitri Mendeleev, and in 2019 we celebrate the 150 anniversary of his original report in 1869.
10. Why do two chemical compounds react?
Chemistry studies changes in matter. A chemical reaction is a process in which one set of chemical compounds are transformed into another. Reaction occur when there is an interaction between the compounds in which some initial bonds are broken and some new bonds are formed.
Why does this happen? In simple terms, because the energy holding the new bonds together is higher than the energy that held the initial bonds. This is the definition of a thermodynamically favored process. Favorable thermodynamics is the most fundamental step that leads two compounds to react with each other. Other drastically important factor is reaction kinetics.
11. What is chirality and where does it come from?
Chirality is a geometric property of certain molecules. A molecule is said to be chiral when its mirror image is not superimposable to the molecule itself. The classical source of chirality in a (organic) compound is the presence of a carbon atom with four different substituents. The concept is far better explained by this basic chemistry youtube video:
The origin of chirality, together with the origin of life is one of the most relevant questions in, not only chemistry, but science in general, so it is not possible to answer in a straightforward manner. The main theory backing it up is based on homochirality, which could have emerged over three steps: mirror-symmetry breaking, chiral amplification and chiral transmission. This is far beyond basic chemistry concepts, but you can further read about it here.
12. What are acids and bases?
Following the original definition by Arrhenius, (1884), an acid is a compound that is able to release a hydrogen cation, or proton (H+). For example, molecules of hydrochloric acid (aqueous HCl) get ionized in solution giving a proton to water, through an acid-base equilibrium:
HCl (aq) + H2O (liquid) ⇌ H3O+ (aq) + Cl– (aq)
HCl in water gives rise to hydronium cations and chloride anions. This is classical acid-base equilibrium.
On the other hand, bases, such as sodium hydroxide (NaOH), can catch protons from water, giving rise to hydroxide anions.
NaOH (aq) + H2O (liquid) ⇌ HO– (aq) + Na+ (aq)
Whereas the Arrhenius acid-base model is very illustrative, it has its limitations, and other models are used to describe more advanced acid-base theories. The most important ones are the Brønsted-Lowry theory (which is a more general version of the Arrhenius theory), and the Lewis theory.
According to the Lewis theory, an acid is a substance that accepts a lone pair of electrons, and a base is a substance that donates a lone pair of electrons. This accounts for acid-base equilibria which cannot be explained by Arrhenius or Brønsted-Lowry theories, such as the basicity of ammonia in water:
:NH3 (aq) + H2O (liquid) ) ⇌ NH4+ (aq) + :OH– (aq)
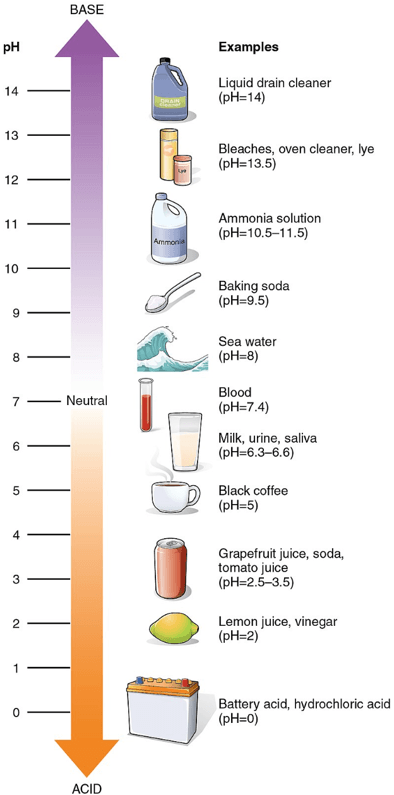
Relative acidity or basicity of solutions or mixtures is measured using a logarithmic scale called the pH scale. It generally goes from 0, most acidic (such as hydrochloric acid solutions), through pH = 7 (considered neutral), up to 14, most basic (such as water solutions of sodium hydroxide). Nevertheless, compounds more basic and acidic than those do exist, pH = 0–14 is definitely not a closed range. Examples of common solutions or mixtures of different pH are shown in the scale below.
13. What is stoichiometry?
Stoichiometry is a very basic chemistry concept. It is just a way of measuring or determining the amount of each substance that is involved in a reaction (reactants), and the amount of products that are generated.
Before actually running a reaction in the lab, a chemist needs to figure out what is the number of molecules of each reagent is required for the reaction to proceed. For this purpose, we use a unit called “mole”. The mole is the base unit of “amount of substance”. One mole accounts for 6.022·1023 molecules. We need it to be a huge number, since there is a huge number of molecules in each gram of any reagent of a reaction.
The stoichiometry of a reaction is the measurement of the relative quantities (or equivalents), measured in moles, of the reactants that are involved in the reaction.
For instance, each 2 molecules (or 2 moles) of hydrogen gas (H2) react with 1 molecule (or 1 mole) of oxygen gas (O2), to generate 2 molecules (or 2 moles) of water (H2O):
2 H2 (gas) + 1 O2 (gas) → 2 H2O (liquid)
So if we were to perform this (rather inpractical, using two expensive, difficult to handle gases, to get a cheap easy to find product such as water) reaction in a lab, we would have to mix together 2 moles of hydrogen per mole of oxygen. This means using 2 equivalents (equiv) of hydrogen respect to the amount of oxygen. In this case, while handling gases, the number of moles of each can be controlled by establishing a partial pressure for each of them.
In case of more common solid reagents, we can consider a hypotetical synthesis of sodium carbionate from carbonic acid. We need 2 equivalents of sodium hydride (NaH) per mole of carbonic acid employed (twice as many molecules of NaH than molecules of carbonic acid).
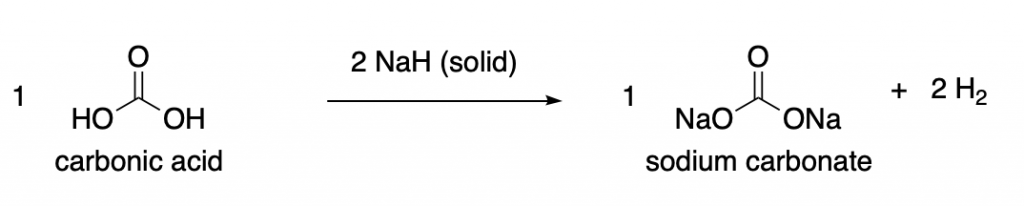
This would yield 1 equivalent, or 1 mole of sodium carbonate, and also release 2 equivalents (or 2 moles) of hydrogen gas as a byproduct.
14. What is oxidation and reduction in chemistry?
Redox processes are a type of chemical reaction in which one of the reacting compounds gets oxidized and the other gets reduced. A redox reaction involves a transfer of electrons. We say that a compound, or atom within a compound, gets oxidized when it loses electrons and the other component gets reduced when it gains electrons.
One of the most typical examples of a redox process is the rusting of iron. Iron metal, Fe0 (oxidation state = 0) reacts with oxygen from air, O2 (oxidation state = 0) to give rust, or iron (III) oxide, Fe2O3.
4 Fe0 (solid) + 3 O2 (gas) → 2 Fe2O3 (solid)
In this new compound, the new oxidation state of iron is +3. Iron has lost 3 electrons, therefore, getting oxidized:
Fe0 → Fe3+ + 3 e–
On the other hand, the new oxidation state of oxygen is -2. Each oxygen atom has gained two electrons, getting reduced:
O2 + 4 e– → 2 O2-
A typical example of a redox process is an explosion in which the explosive compound gets oxidized violently. C4 is a common plastic explosive, much more energetic than dynamite. The main component of C4 is RDX (Research Development Explosive), also known as cyclonite or, according to IUPAC, 1,3,5-trinitro-1,3,5-triazinane.
The process of oxidation of RDX is thermodynamically favorable, and gives rise to a exothermic reaction, in which a large amount of energy is released in the form of heat and light, causing the explosion.
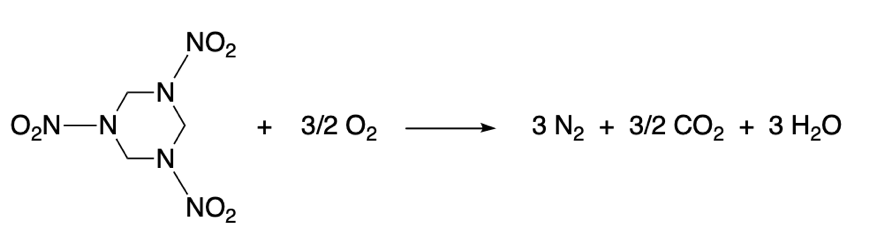
The N–NO2 bonds are extremely unstable and prone to oxidation. That’s what makes this kind of compounds highly explosive.
15. What is radioactivity?
Radioactive decay, or radioactivity, is basically a process in which an unstable nucleus loses energy by the emission of radiation in the form of a particle. This concept is clearly explained in the following video:
This is more of a physics concept than a basic chemistry concept, but it is very relevant for chemists.
Not all atoms that exist are stable, some are what you could call “not meant to be”. Most of the matter that we see is made up of combinations of protons, neutrons that are stable (stable atoms, specifically, stable nuclei). But other combinations of neutrons and protons give rise to unstable nuclei, that eventually fall apart. This is the basis of radioactivity in simple terms.
During the decomposition of these unstable atoms, energy is released in the form of particles. This release of energy can be detected, and it is what we call radiation. When this process takes place, a new nucleus is formed, and therefore, a new atom. This new atom can be also unstable and keep releasing radiation until it turns into an stable atom, which no longer emits energy as radiation.
Finally, I want to make an update with a 16th concept! This deserved a separate post, and here it is: A tutorial review on why chemicals react! If you are serious about learning chemistry, you need to master the basics behind the concepts of thermodynamics and kinetics.
To wrap up, I hope you all (or your students, or children) got something out of this. As I mentioned above, this is just a simple introduction to many basic chemistry concepts. If something is not clear enough, or want me to further develop or update any particular point, please, make sure to let me know.
If you are using this article to learn or teach high school chemistry, we have reviewed now the best books to learn chemistry at that level. You can check it out here.
A perfect tool to complement these theoretical concept, especially for young students, is getting a nice chemistry kit.
Besides, most concepts described here are based on models. You need to clearly grasp the idea that all models are wrong in science, so make sure to check out this other reading.
Also, go ahead and share this article with your students or whoever is learning chemistry!

This is very basic concepts which we have to clear in our mind….tq. U to this website ❤️
Glad to help!
Thanks for this.
Thank you for reading!