The most important family of reactions that you will study in introductory organic chemistry are substitution reactions. The difference between SN1 vs SN2 types of reactions is a key concept, and it is based upon a key question: what is steric hindrance?
If you an organic chemistry student, you definitely need to get a grip on the two general types of substitution reactions. And if you are teaching a basic o-chem course, you need to make sure your students master them. Also make sure to be aware of the reasons behind why molecules react with each other!
Most organc chemistry courses (check here the best books) start off by checking out substitution reactions.
So you are probably familiar with them. They are key for answering the question of what is steric hindrance in organic chemistry.
In any case, let’s cut right to it and quickly explain what substitution reactions are.
What are Substitution Reactions?
Substitution reactions are chemical reactions in which a functional group on a molecule is replaced by another one. One of the most basic examples of substitution reaction is the Finkelstein reaction.
The Finkelstein reaction is also known as the “halogen exchange” reaction, because that is basically it:
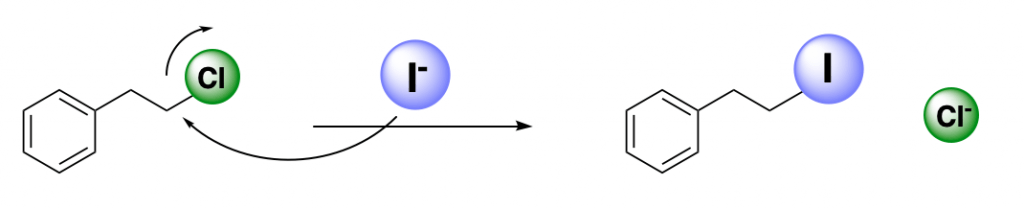
A carbon-halogen bond is polarized, the carbon atom attached to the halogen is electrophilic, and can be attacked by an external nucleophile.
We have covered this concept of electron distribution in a previous post. Halogens can act as nucleophiles. This results in exchanging one for another, through an SN2 mechanism.
This halogen exchange reaction is actually an equilibrium: it is reversible and it can take place in both directions. However, you can drive this kind of substitution taking advantage of solubility:
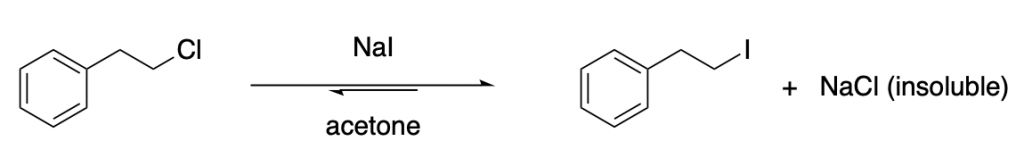
In the example above, sodium chloride, which is significantly insoluble in acetone, precipitates out of the reaction mixture. This drives the equilibrium to the right. This a nice visualization of Le Chatelier’s principle.
This substitution reaction goes through what we call a SN2 mechanism.
SN2 vs SN1 Mechanisms
In organic chemistry, a reaction mechanism is the step by step sequence in which a reaction takes place. It covers the way the reactants are joined up together through transition states, and how they transform into the reaction products.
A simple substitution reaction can go through two basic types of sequences, or reaction mechanisms: SN2 vs SN1.
S stands for substitution (which we already covered), N stands for nucleophilic (because a nucleophile is exchanged for another one).
1 and 2 stand for unimolecular and bimolecular, respectively. These are simple mechanistic concepts,
How Do SN2 Reactions Take Place?
Bimolecular reactions, such as SN2, take place through a transition state in which the two reactants are joined together.
In the following example, the electrophile (in this case ethyl bromide) and the nucleophile (a hydroxyl group) react through a pentacoordinate transition state which involves both reactants:
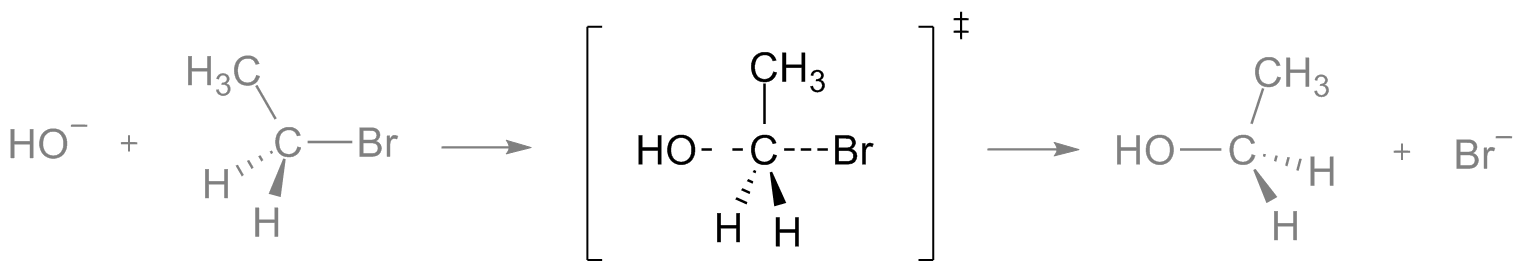
This has different practical implications.
For example, since two of the reactants are involved in the rate-limiting step of the overall process, the rate of the reaction will depend on the concentration of both reactants.
Furthermore, substitution reactions that go through a SN2 mechanism, go through an inversion of configuration (R to , or vice versa) in the carbon atom in which the exchange takes place. This is because, as you can see in the scheme above, SN2 reactions go through a “backside attack” substitution.
But some substitution reactions cannot go through this kind of SN2 mechanism. This is mainly because of steric hindrance.
What Is Steric Hindrance?
Look at the diagram below. This kind of bimolecular attack that defines SN2 reactions can take place easily in primary electrophiles, or even on secondary ones.
However, tertiary electrophiles such as tert-butyl bromide cannot undergo this kind of backside attack. This is because of steric hindrance.
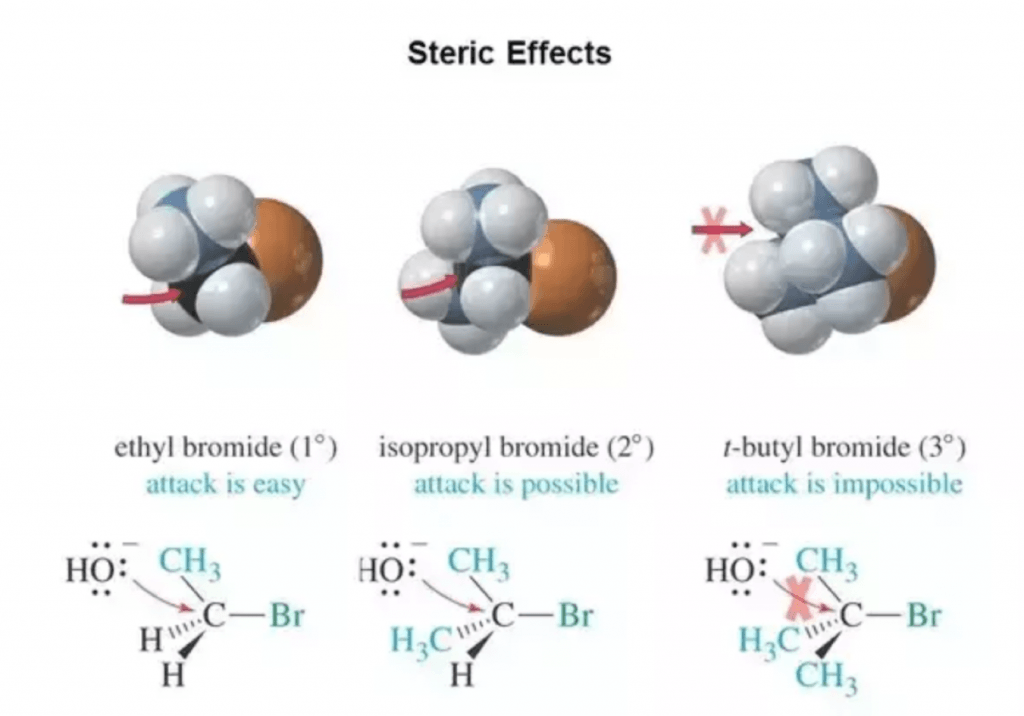
We have previously covered on this post both electronic and steric effects in organic chemistry. Make sure to check it to expand further on this topic.
But substitution reaction can actually take place in bulky electrophiles such as tert-butyl bromide, only not through a SN2 mechanism.
By the way one of the coolest ways to actually visualize steric effects, and what the SN1 vs SN2 difference is all about, is using a molecular model such as these! Make sure to also check ur visual guide on how to use molecular models for learning organic chemistry.
SN2 vs SN1 is a key concept that anyone that’s getting into chemistry, not only in organic. In case you are getting started, and preparing you AP chemistry exams, maybe you want to get your hands into some prep material.
Steric Hindrance and the SN1 Mechanism
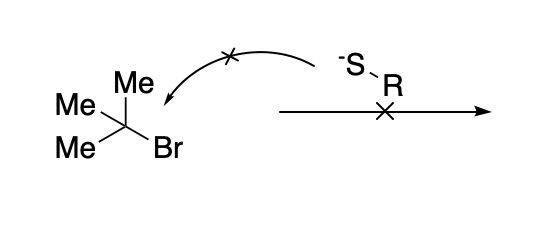
The SN1 is a substitution reaction mechanism in which the nucleophile does not attack the starting electrophile directly.
Instead, since steric hindrance prevents this from happening, the reaction takes place in two different steps: First, the leaving group “detaches” from the electrophile, giving rise to a transient carbocation.
This new electrophile is much more accesible for the nucleophilic attack, and can be attacked by an external nucleophile.
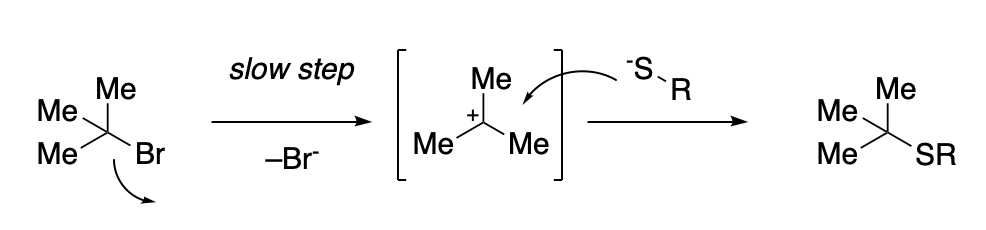
Since this transient intermediate is cationic, it will highly electrophilic, which means that the combination with the nucleophile will be very fast. This makes the formation of the cationic intermediate the slow or rate-limiting step of the process. This step involves only one of the reactants, that’s why it is called a “unimolecular” (SN1) reaction.
It is worth mentioning that the high stability of tertiary carbocations, due to inductive effects, is a key element that allows for this pathway to be feasible:
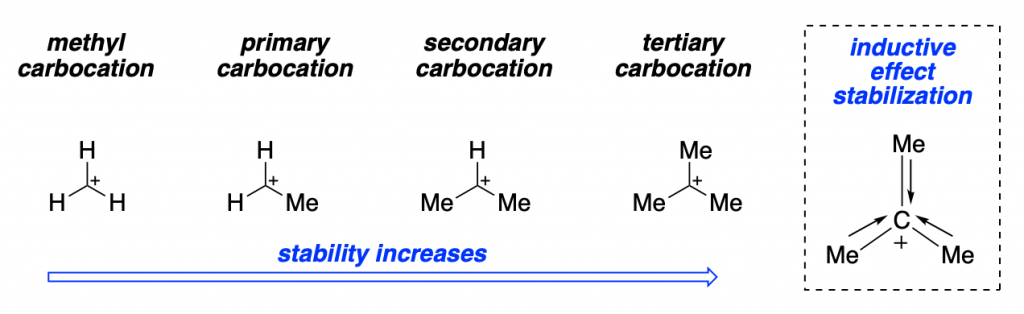
The fact that SN1 reaction go through a cationic intermediate has the opposite consequences than for SN2 reaction: the reaction rate depends only on the concentration of the electrophile.
Stereochemical Outcome of SN1 Substitution Reactions
Besides, instead of inversion of configuration at the electrophilic carbon, what you get is a 50:50 mixture of both R and S enantiomers. This happens because the trigonal planar cationic intermediate can be equally attacked from both sides.
This is illustrated in the following scheme:
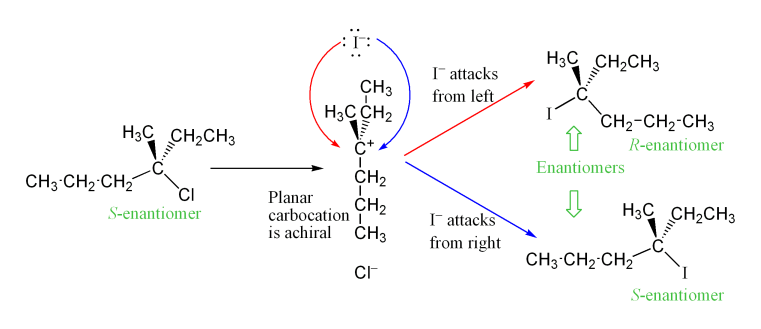
Starting from a single S enantiomer of a tertiary electrophile, if we perform a nucleophilic substitution, due to steric hindrance, it will take place through a SN1 mechanism. A planar carbocation will form, which can be attacked equally from both sides. This will result on a 50:50 statistical mixture of S and R enantiomers.
To Sum Up: SN1 vs SN2 Mechanisms According to Steric Effects
In short, substitution reactions are simple exchanges of functional groups, such as different halogens.
If steric effects allow it, these reactions take place through SN2 bimolecular concerted mechanism, which gives inversion of configuration.
If steric hindrance is too high for this to happen, substitution reactions take place stepwise. First, the leaving group detaches from the electrophile, giving rise to a planar cationic intermediate. This is more sterically accessible, and can be attacked by the nucleophile, from either side equally.
Don’t miss our general guide on how to learn chemistry for an overview of what we consider the best approach, as well as many resources.
This pretty much sums it up.
We hope everything ended up being clear, but if you have any question or suggestion, make sure to reach us in the comment section.
Furthermore, we encourage you to share this article with whoever might find it helpful, especially students or professors!

Very well explained, but I think you overlooked the fact that tertiary carbocations are more stable, which allows the formation of the carbocation by “detaching” the leaving group from the electrophile. I don’t know, I think this is important information to help identify which one is which.
Cheers.
Thanks for pointing it out. I think that fact is worth mentioning, I will update it as soon as I have time.
Very funny I like this information