Wherever we look around, you can see both inorganic and organic compounds. When we go to supermarket, we see different items; some are organic and others are inorganic. What is the difference between organic and inorganic compounds?
Well, the chemical difference is not the one you hear on the news which distinguishes “organic” vegetables from “non-organic” ones. Guess what, both are made up of organic and inorganic compounds.
Let’s say that the “agriculture industry” definition is not the same as the chemical definition. In chemistry, there is a major difference, which is well defined.
Telling the difference between organic and inorganic compounds is one of the main things you need to make clear while learning chemistry. If you are interested, learn more about thermodynamics and kinetics, another two of thee most important concepts in chemistry.
In this article we will explain it in detail, so at the end you will be able to differentiate both of types of chemicals without any difficulty. We will try to solve all your doubts about this eternal chemistry question!
In the early days, scientists separated organic and inorganic compounds on the fact that the first group was considered as a result of the activity of living beings, whereas the second group belonged to the processes unrelated to any way of life. Now there are much clearer definitions.
| Did You Know? Inorganic and organic chemistry are two of the main disciplines of chemistry. Organic is related to (most of ) the chemistry of carbon, and inorganic chemistry studies basically the rest of chemical compounds. |
Introduction
About 200 years ago, at the transition between alchemy and chemistry, chemists classified the chemical compounds into two main groups.
1. Organic Compounds
An easy, layman-friendly definition for organic compounds is that those are the ones which are derived from living things such as plants and animals are known as organic compounds like sugars, lipids, proteins, nucleic acids, etc.
More strictly speaking, we consider a compound to be organic if it is made of carbon atoms which participate in covalent bonds. Generally (but not always), organic compounds also present covalent C–H bonds.
2. Inorganic Compounds
An easy definition for an outsider, is that those compounds which are obtained from non-living things or mineral sources are known as inorganic compounds like NaCl (table salt) and NaHCO3, (baking soda), etc.
Defining inorganic compounds is pretty easy after having defined organic compounds. As a rule, every chemical that does not fall into the category of “organic”, is considered an inorganic compound.
The Vital Force Theory and the First Chemical Total Synthesis
Let’s go back in time once again, to the very early days of chemistry. The theory known as the “vital force theory” might ring a bell to you if you are familiar with the history of chemistry.
This theory was proposed by Swedish chemist Berzelius in 1815. This theory states that organic compounds can’t be synthesized in a laboratory. Early chemists believed that organic compounds could only be obtained from living organisms, through “vital forces”. That is why this theory is referred to as “vital force theory”.
In 1828, Friedrich Wohler, a German chemist, synthesized urea in the laboratory. This accounts for the first chemical total synthesis of a natural organic compound ever!
This accomplishment showed that it was possible to synthesize an organic compound (urea), starting from an inorganic compound (ammonium cyanate), in the laboratory: treating silver cyanate with ammonium chloride afforded a crystalline compound that was found to be identical to urea isolated from urine.
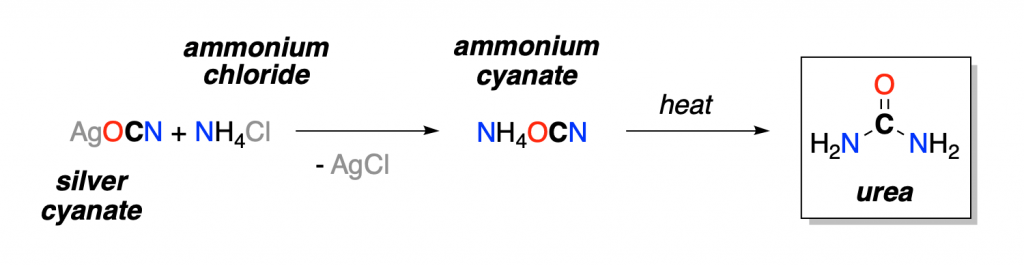
This chemical transformation invalidated the vital force theory, and soon after this, chemists began to make organic compounds in the laboratory. Hence the modern definition of organic compounds was introduced in the scientific world. This also marks the very beginning of organic chemistry as a discipline.
The Modern Definitions
Organic Compounds
The compounds which contain carbon atoms as main constituent, which are bonded together through covalent bonds, are called organic compounds. Most organic compounds also contain hydrogen. Other common elements present in organic compounds are oxygen, nitrogen, sulphur, halogens, or phosphorous. But those are not the only ones.
In most cases, all atoms of the different elements are held together through covalent bonds. Some exceptions would be, for example, organic carboxylates, or ammonium salts. But you could argue that those are “inorganic salts of organic compounds”.
Some compounds that might sound “non-organic as hell” such as polymers (a fancy name for plastics), are actually long-chained organic compounds. An example is polystyrene. It’s backbone is basically all covalent C–C and C–H bonds.
Bear in mind that “organic compound” does not imply “biochemical compound”. On the other hand, the backbone of biochemistry is mostly organic compounds (although metals are extremely important in biological systems such as iron in hemoglobin).
Inorganic compounds
Take every organic compound out. You are left with inorganic compounds. If it doesn’t fall into the definition of organic, it is inorganic.
In general, the compounds which do not have C–C or C–H covalent bonds are called inorganic compounds.
There are many compounds that only have covalent bonds, they have carbon atoms, but are not organic compounds. Examples of this type of inorganic compounds include carbon monoxide, carbon dioxide, inorganic carbonates, carbides, etc. Notably, allotropes of carbon such as graphite, graphene or diamond, contain only carbon atoms, but are considered inorganic compounds.
As you can see, sometimes the definition is not so well established. In fact, I couldn’t really find a clear definition for both provided by IUPAC. This illustrates the fact that defining the line between inorganic and organic chemicals.
Some interesting examples of this middle ground are organometallic compounds. These are made up of an organic component, generally bound to an inorganic component through a carbon–metal bond. These are really fun and are one of the most widely explored research topics in modern chemistry!
Major Differences Between Organic and Inorganic Compounds
We will try to sumarize in a quick comparison table the key differences between organic and inorganic compounds.
However, bear in mind that in most cases these are just generalizations and won’t be true for any scenario, and definitely will have exceptions.
| Organic Compounds | Inorganic Compounds | |
| Definition | Organic compounds are generally constructed by a backbone made of C–C and C–H covalent bonds. | Inorganic compounds do not have a backbone based on C–C covalent bonds. |
| Occurrence | Living organisms are generally made up of organic compounds, for the most part. | Inorganic compounds are generally found in non-living entities, such as minerals, the air, or outer space. |
| Composition | Organic compounds are mainly made up of carbon and hydrogen, but also oxygen, nitrogen, halogen, phosphorus and others. | Inorganic compounds can contain a vast amount of chemical elements. There are inorganic compounds for every element of the periodic table. |
| Chemical Bonding | Organic compounds are held together by covalent bonds. | Inorganic compounds form ionic bonds, or metallic bonds, although covalent bonds may also be present. |
| Solubility | In very general terms, organic compounds are less soluble in water. They are usually soluble in organic solvents. | In very general terms, inorganic compounds are soluble in water and less soluble in organic solvents. |
| Melting Point | In very general terms, organic compounds have low melting and boiling point. | In very general terms, inorganic compounds have high melting and boiling point. |
| Examples | Carbohydrates, lipids, proteins, nucleic acids, organic solvents, methane… | Sodium chloride (table salt), graphite, metallic iron, steel, glass, carbon dioxide… |
And as you can probably guess, the examples for both types of both types can go on forever.
Examples of Organic Compounds
Time to dive into learning organic chemistry! These are just some natural and non-natural examples of organic compounds.
Carbohydrates
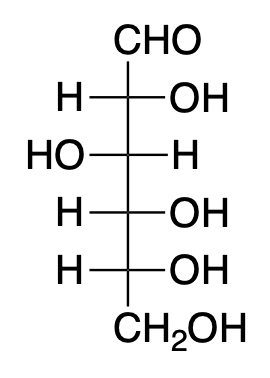
These are commonly known as sugars. In terms of functional groups, these are aldehydes or ketones having additional hydroxyl groups. Carbohydrates are a simple way to illustrate organic compounds, since they are just chains of C–C and C–H covalent bonds in the company of some of the most typical organic functional groups (alcohols and carbonyls). Examples of carbohydrates are glucose, fructose, sucrose, etc.
Proteins
Proteins are made up of chains of amino acids joined together to form peptides. Proteins are actually polymers, which can be made up of a single chain of many amino acids, or of several chains that are packed together by non-covalent interactions. Since they are made of amino acids, they contain carbon, hydrogen, oxygen, and also nitrogen atoms, everything held together by covalent bonds, and also non-covalent interactions. A classical example of proteins are enzymes.
Organic Solvents
Organic solvents are organic compounds which are commonly used to dissolve chemicals in the lab, mainly for setting up chemical reactions. “Like dissolves like” they say, so these solvents are a must for carrying out organic reactions. They are usually simple organic compounds made of carbon, hydrogen, and also oxygen or nitrogen, sometimes sulphur. They are usually liquids at room temperature and have boiling points ranging from 40 ºC to 200 ºC. Common examples are hexane, cyclohexane (CyH), acetone, tetrahydrofuran (THF), toluene (PhMe), ethanol (EtOH), methanol (MeOH), benzene (PhH), dimethylsulfoxide (DMSO) or dimethylformamide (DMF).
Whatever Organic Compound that You Can Imagine Making on an Organic Chemistry Lab
The only limit for organic compounds is the imagination of the chemist. Theres is most likely an infinite number of combinations in which you can arrange carbon and hydrogen atoms to form organic compounds. Not to mention other elements.
That’s something I just made up in less than 1 minute in ChemDraw, and it seems like a totally reasonable organic compound.
Examples of Inorganic Compounds
Getting ready to study the realm of inorganic chemistry? These are just some common examples of inorganic molecules.
NaCl – Sodium Chloride or Table Salt
The salt you use for cooking is mostly sodium chloride, NaCl, and this is the most classical example of an inorganic compound. Specifically, it’s an ionic compound composed of an equal number of sodium(I) cations and chloride anions, arranged though a symmetrical three-dimensional network.
Carbon dioxide
Carbon dioxide is another example of inorganic compound with a chemical formula CO2. Despite of the presence of carbon atom, CO2 is considered an inorganic compounds because containing carbon and covalent bonds doesn’t directly make a compound organic. You need a C–H bond or an equivalent.
For example, carbon tetrachloride, CCl4, is considered an organic compound, because instead of C–H covalent bonds it has C–Cl bonds, which are electronically equivalent. The bonding model in carbon dioxide, carbon monoxide, and other small inorganic compounds is quite different.
Diamond and Graphite
Allotropes of carbon such as graphite, graphene or diamond are classified as inorganic compounds, even when they have
Example of an Organometallic Compound
Right in the middle of organic and inorganic compounds, we can find organometallic compounds, which are characterized by having a carbon–metal bond (which in many cases is a “hybrid” between a covalent and an ionic bond).
An example of this are Grignard reagents (such as phenyl magnesium bromide) or organolithium compounds (such as butyl lithium).
To Sum Up
I hope we managed to explain clearly the basic differences between organic and inorganic compounds.
Organic compounds always contain carbon atoms, and almost always hydrogen atoms, all of them held together by covalent forces.
Inorganic compounds are just the rest!

That was Explained very Nicely, I can see you have given a lot of time to the writings. After reading this any student can know the basic differences between organic and inorganic compounds. just missing one thing You can use more images to make it more attractive to viewers and also that will help you in explaining easily. I loved the different examples you have shared, that really helps a lot. fantastic job.