Today we will discuss an awesome chemical natural wonder: crystal caves. Crystal caves are natural formations based mainly on inorganic crystals, such as selenite, or hydrated calcium sulfate, some of the largest natural crystals ever found.
You can find this formations in different places at the US, for example, there is a crystal cave in Wisconsin, a crystal cave in Ohio and a crystal cave in California. Besides, one of the most impressive ones is located in Naica (Mexico).

We will first explain the chemistry behind these natural marvels and then check the best crystal caves that you can find and how to visit them. As a chemist, I find them as a source of inspiration.
- What Are Crystal Caves and How Do They Form?
- The Great Cave of Crystals in Mexico
- Visit the Crystal Cave, Wisconsin
- Visit a Crystal Cave, Ohio
- The Crystal Cave in CA: Sequoia National Park
What Are Crystal Caves and How Do They Form?
Many inorganic compounds can form crystals naturally. I spent part of my youth growing crystals as part of chemistry home experiments, and now as a professional chemist, I also have to it on a weekly basis. Crystallisation is an important process in chemistry, but it is nothing new that we discovered in the lab. Nature has been providing examples of growing crystals since forever. And it does it pretty well.
There are different materials that can give rise to crystal caves. Some examples are speleothems (“cave deposits” from Greek). These are deposits of minerals such as limestone (calcium carbone) or dolomite (mixed calcium and magnesium carbonate).
If you are not familiar with these terms, maybe you are interesting in some resources for learning inorganic chemistry.
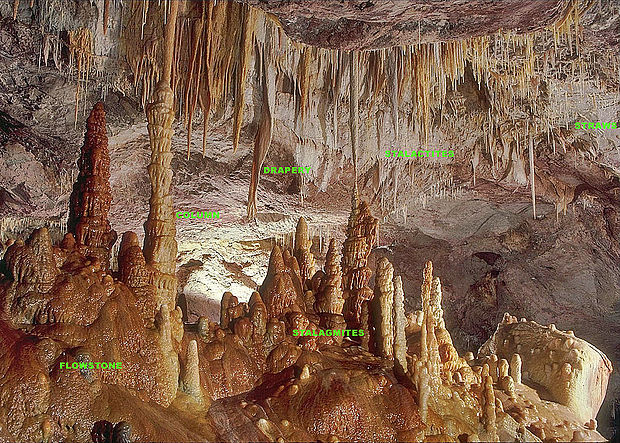
But this simple deposits don’t give rise to large crystals, they are just microcristaline solids. The best example of a cave with huge crystalline formations is the crystal cave in Naica (Mexico).
Natural Large-Scale Crystal Cave Growing
As you may know, one of the best ways to grow crystals in the lab is using a hot solvent to obtain saturated solution of your compound. Then you let the saturated solution cool down, and if you are lucky, crystals will eventually crash out. But no fancy Nobel-prize wining chemist invented this. Nature did!

Millions of years ago, the caves were filled with water rich in hydrated calcium sulfate (gypsum), in a form called selenite, which gives rise to colorless-white crystals (if want to learn more, you may want to take a look at some of the best inorganic chemistry textbooks). At that time, these waters were heated by magma, above 60 ºC. Over thousands of years, as Earth cooled down, these saturated solutions also got below this temperature. This caused the calcium sulfate to start nucleating and the crystals began to grow. This is a textbook definition of crystallisation by extremely slow cooling, and you can find more details in this original report in Geology. Remember, do your crystallisations slowly (maybe not as slowly as Nature) for them to succeed!

The Great Cave of Crystals in Mexico
Out of all crystal caves, the one located in Naica (Mexico) is definitely the most impressive one. The main chamber contains some of the largest natural crystals ever found. These crystals are mainly selenite, and its formation was described in the last section. The largest weights 55 tons and has 12×4 m dimensions. Simply impressive. The scenery seem like taken right out of a science fiction movie or videogame.

Unfortunately, there are downsides associated with this Mexican wonder. The cave is extremely hot, on average between 45 and 50 ºC, up to a maximum of 58 ºC, with a roughly 100% of humidity. Therefore, visit is limited to authorised trained scientists. Using special suits, it is possible to stay down there no longer 10-15 minutes. These factors result in this crystal cave being relatively unexplored after its discovery in 2000.
Besides, the fact that the crystals are made of hydrated calcium sulfate, makes them very sensitive. The atmosphere saturated in water of the cave allows them to survive, but taking them out of the cave leads to dehydration and subsequent loss of crystallinity.
Maybe it is better for the crystal cave of Naica it to remain fairly unexplored. But don’t worry! There are several nice options across the US to visit a crystal cave, with not-so-extreme conditions.
Speleothem Crystal Cave, Wisconsin
In Pierce County, you can find the crystal cave in Wisconsin. It was discovered much longer ago, in 1881 by W. R. Vanasse. The crystal cave in Wisconsin is filled with speleothems, mostly in the forms of stalagmites, stalactites or columns.
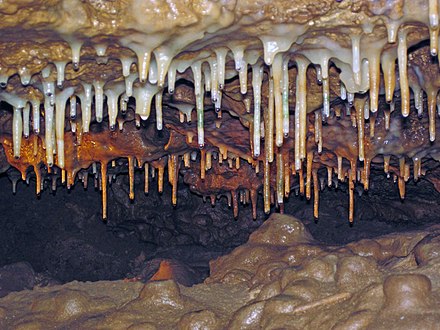
An interesting fact is that this caves were designated as public bomb shelters in 1942. The cave is so massive that it was the only one able to fit the whole population of the town which was using it as a protection element. The Wisconsin crystal caves can be visited any time of the year except for the winter.
Strontium Crystal Cave, Ohio
The next crystal cave, Ohio, is a cave made mainly of limestone (calcium carbonate) and you can find it in Put-in-Bay, in Lake Erie. It was discovered in 1897, and only since 2016, you can visit it!

In this crystal cave you can see crystals of up to 1 m long. These crystals are made of a mineral called celestine, which is a form of strontium sulfate. Strontium salts, specifically strontium carbonate and nitrate, are widely used to make fireworks. The strontium ion gives red fireworks its color. For this reason, the space within this cave is significantly bigger now than when it was discovered. This means more room for visiting and also fireworks. Yay.
The Crystal Cave in CA: Sequoia National Park
You can also find a crystal cave in California, within the magnificent Sequoia National Park. There are more than 200 caves in this National Park, but this one is worth a visit. This cave is usually open for visits from the end of May to the end of September. The crystalline formations in this crystal cave are mostly based in limestone speleothems, so nothing new chemically, but the scenery make it worth checking out if you are a geology enthusiast.

Keep in mind that if you want to visit this crystal cave, you will have to buy the tickets online in advance at the Foothills or Lodgepole Visitor Center, they are not selling them on-site.
As you can see, Nature outranks us chemists in many tasks. Not only builds up extremely complex organic molecules without much effort, but Nature sure also beats us growing crystals! However, to be fair, Nature had millions of years to adapt, but modern science is barely a couple of centuries old. I guess we are on the right path.
Be sure to let us know in the comments which kind of natural wonders would you like to see chemically-explained in the future!

My personal experience with crystals has been through growing them as part of chemistry experiments. Although growing crystals in the lab can be exciting, nature provides us with the most impressive examples. I am fascinated by the fact that nature has been growing crystals for millions of years and that the process is a textbook example of crystallization by extremely slow cooling. Crystal caves are an impressive natural wonder that is formed by inorganic crystals. These caves can be found in different locations around the United States, such as Wisconsin, Ohio, and California. However, the most stunning crystal cave is located in Naica, Mexico. The cave has huge crystals that are made of selenite, or hydrated calcium sulfate, and were formed by crystallization caused by extremely slow cooling. Despite its beauty, the cave is relatively unexplored due to its extreme conditions. I find these formations a source of inspiration. Have you ever visited a crystal cave? What was your experience like? Do you have any recommendations for other crystal caves to visit?