If you are starting to face basic organic chemistry, you may feel overwhelmed with the huge amount of material.
Introductory organic chemistry courses can seem like the most difficult ones. But don’t worry! In this post we are going to talk about a “trick” or key concept that will help you along the way.
Let’s say, this concept is a “point of view” that you can adopt, and which will help you see organic chemistry from an easier perspective. See the big picture.
What Are the Keys for Learning Organic Chemistry?
You are not alone feeling like this subject is special. This makes complete sense, since organic chemistry is a very unique branch of science. The learning process that your brain must follow for o-chem is very different.
In any case, you really need to be prepared. To do so, a good organic chemistry textbook and a nice molecular modeling kit are gonna be your best companions. You obviously also need a solid background on general chemistry.
And yeah, learning how to run a perfect TLC helps too!
However, there are key concepts, specifically the one(s) we will discuss here, that will help you see organic chemistry from a brighter side.
Keep reading!
What Is the Most Important Concept to Grasp in Basic Organic Chemistry?
In a very simplefied fashion, you can argue that you can explain everything in organic chemistry by arguments of either “electronic effects“, “steric effects“, or a combination of the two.
In very simple terms, nucleophiles attack electrophilic positions due to electronic effects. More accesible electriphilic centers will be more reactive, due to steric effects. This simple but important concepts are the basis of most rationalizations or models in organic chemistry.
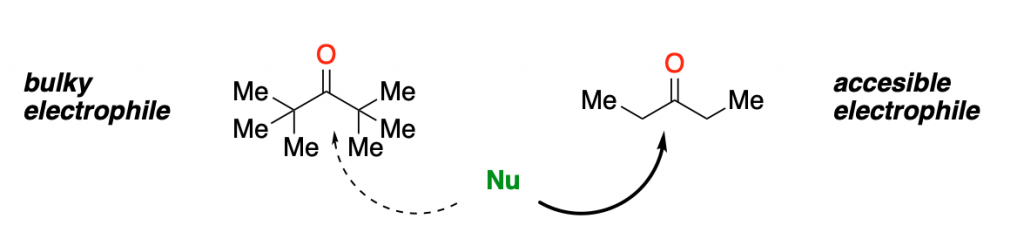
Stereoelectronics is not a concept that, by itself, is usually taught in introductory courses (if you check the Wikipedia page on it, for example, it shows a significantly advanced discussion). However, we like to think about a combination of steric and electronic effects as a simplified version of this concept. This combination is key for grasping the big picture of organic chemistry.
We will explain what this combination is about, going through the basics of what electronic and steric effects are. We will show different examples on how these two are used to rationalize chemistry, both independently or in combination.
I would say that most textbooks are missing a specific general overview about this (although you can argue that this is the entirety of basic organic chemistry itself). So, we will try to cover that gap on this article.
Basic Organic Chemistry: What are Electronic Effects?
If you have studied anything about reactivity of organic molecules, you know that the movement of electrons rules everything.
Generally, two organic molecules react because one part (or functional group) of one of the molecules has with high electron density (nucleophile) and another part of another molecule, has a low electron density (electrophile).
What Are Nucleophiles and Electrophiles?
In very simple terms: we say that a molecule behaves as a nucleophile if it has a functional group that has a lot of electrons (high electron density), and wants to attack another functional group that lacks electrons. This second partner, which has low electron density and wants electrons, is called electrophile.
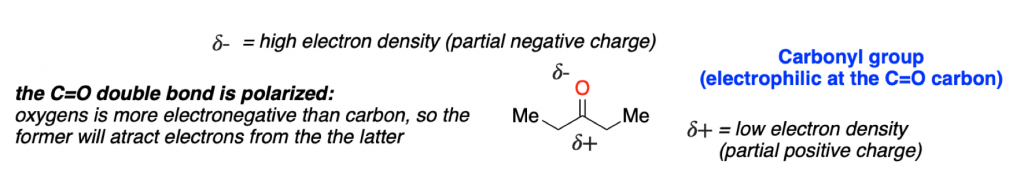
The previous figure has a scheme of the most typical electrophile in organic chemistry: a carbonyl group.
This is a great example of how electronics work. In this molecule, a electronegative atom (O) is covalently attached to a carbon. Oxygen is more electronegative than carbon, so it wants to hold the electrons of the covalent bond more than carbon. This is a combination of induction and resonance effects.
But the result is clear: a partial negative charge (𝛿-) is generated at the oxygen atom, and a partial positive charge (𝛿-) is generated at the carbonyl carbon atom. This charge distribution, makes the carbon atom of the carbonyl group a potential electrophile that can react with different nucleophiles.
How Does Charge Distribution Result on Reactivity Trends?
Plus and minus want to get together. In this way, nucleophiles can react with electrophiles.
Typical nucleophiles for ketones are organic or organometallic reagents with high electron density. In this example, a negatively charged carbon atom of a Grignard reagent, such as PhMgBr (phenyl magnesium bromide), acts as a nucleophile.
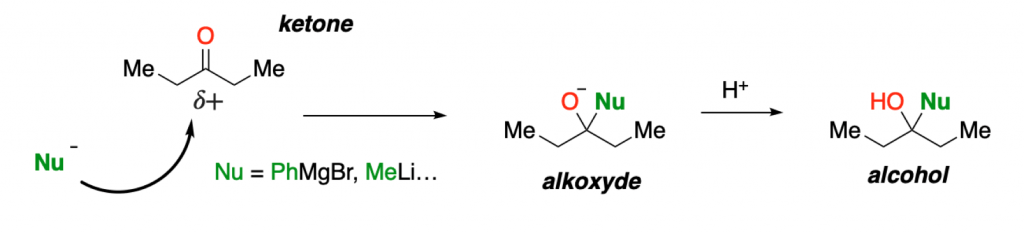
The addition of a nucleophile to a ketone releases this charge polarization on the electrophilic ketone (this is the driving force of the reaction), giving an intermediate alkoxyde, which after treatment with a proton source (H+), gives the corresponding tertiary alcohol.
This is probably one of the first examples that you will be taught in any organic chemistry reactivity course, and it’s really simple. Isn’t it?
Well, good news! Most reactions between organic compounds can be explained by just using his principle of electron distribution!
So, how do we determine were are the positive charge and the negative charge in each organic molecule, so we can know what will react with what?
Let’s explore general trends in electron density. Keep reading!
Electron Density Maps of Molecules
This density of electrons within a molecule can be mapped in a very visual manner. Take a look at some basic examples in the figure below:
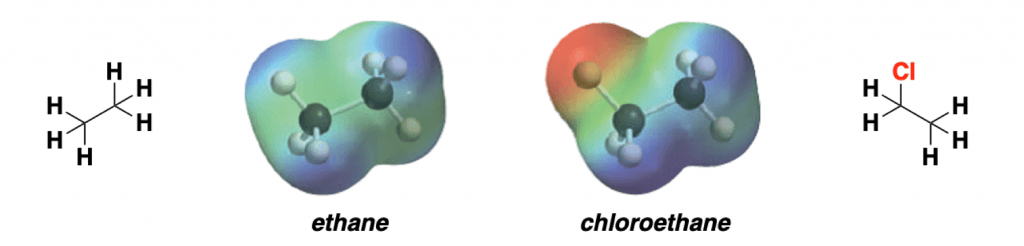
The first example is a simple hydrocarbon: ethane. Ethane is a very apolar molecule. Their electrons are distributed evenly throughout the molecule. There is no charge polarization, so it will be an unreactive molecule.
On the other hand, chloroethane is a highly polarized molecule. As you can see in the density map, there is a lot of electron density (red zone) in the chlorine atom. This is because chlorine is more electronegative than the carbon it is attached to, generating a partial positive charge on the adjacent carbon.
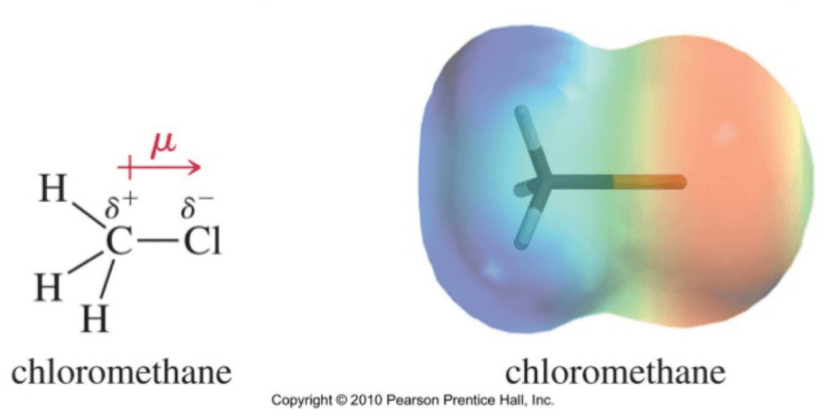
Exactly the same happens with chloromethane or, in general, any organic alkyl halide. This controls completely the reactivity of this type of molecules. Learning how to tell where the electrons are is one of the most important things to grasp!
Electronics-driven Substitution Reactions
This bond polarization makes the carbon directly attached to the chloride an electrophilic center. Electrophilic centers such as this one, can be attacked by nucleophiles through a nucleophilic substitution reaction (SN2).
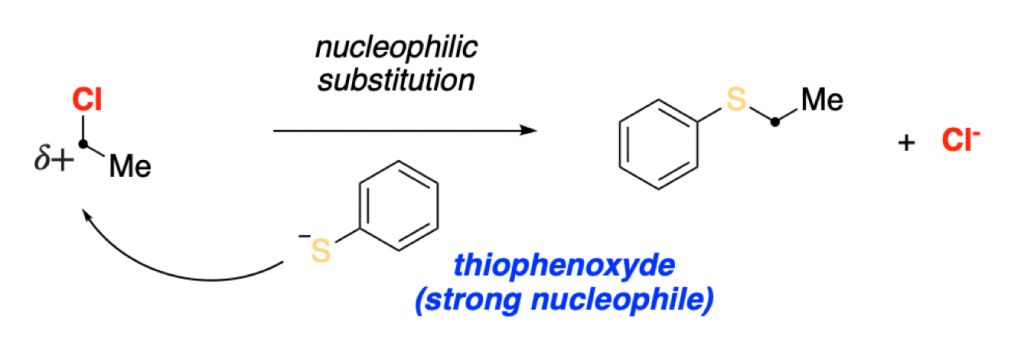
Nucleophilic substitution reactions and electrophilic additions, together with elimination reactions, are generally all the reactivity covered in any introduction to organic chemistry courses.
To further dive into the concept of electron density, let’s look back at our first example in the previous section. Another density map can easily explain the reactivity of a carbonyl group:
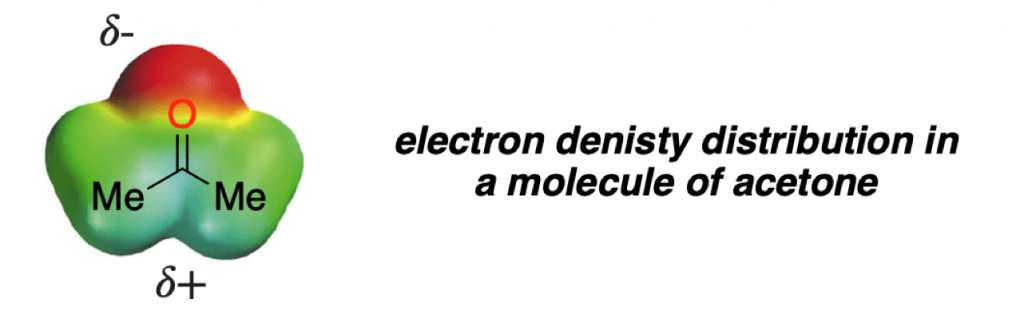
As you can see, the highly polarized bond gives rise to a partial negative charge in the oxygen (red zone) and a partial positive charge in the C=O carbon (blue zone).
This is a simple concept, but it really does help seeing the bigger picture of organic chemistry. If you understand that organic reactions happen because high electron density zones of molecules “attack” low electron density zones of other molecules, that is big deal!
Another great example of electronics-driven organic reaction is electrophilic aromatic substitution. However, we will look into into it at the end of this article, as it also works as a great example of a combination of electronic and steric effects.
But first, we need to look at the other side of the coin. Steric effects, or steric hindrance.
What are Steric Effects?
Imagine organic compounds as big clusters of electrons.
Electrons are negatively charged, so generally, there is an energy penalty on one molecule approaching another (although there are exceptions of positive non-covalent interactions).
Imagine two negative poles of two magnets: they stay away from each other, unless a bigger force, puts them together.
This is the same with organic molecules. On this case, as we saw on the section about electronic effects, this bigger force that can put electrophiles and nucleophiles together is electron polarization. The higher thermodynamic stability that is acquired while putting together an electrophile and a nucleophile, overrides the natural repulsions between molecules.
A similar concept is illustrated in a potential energy diagram. A system of two atoms acquires the minimal energy (the most stable situation) at a given bond distance. If you pull them closer together, electronic repulsions get too big (moving towards the left on the x axis), and the energy of the system rises. This means basically that it gets destabilized because of nuncleus-nucleus (+) or electrons-electrons (-) repulsions.
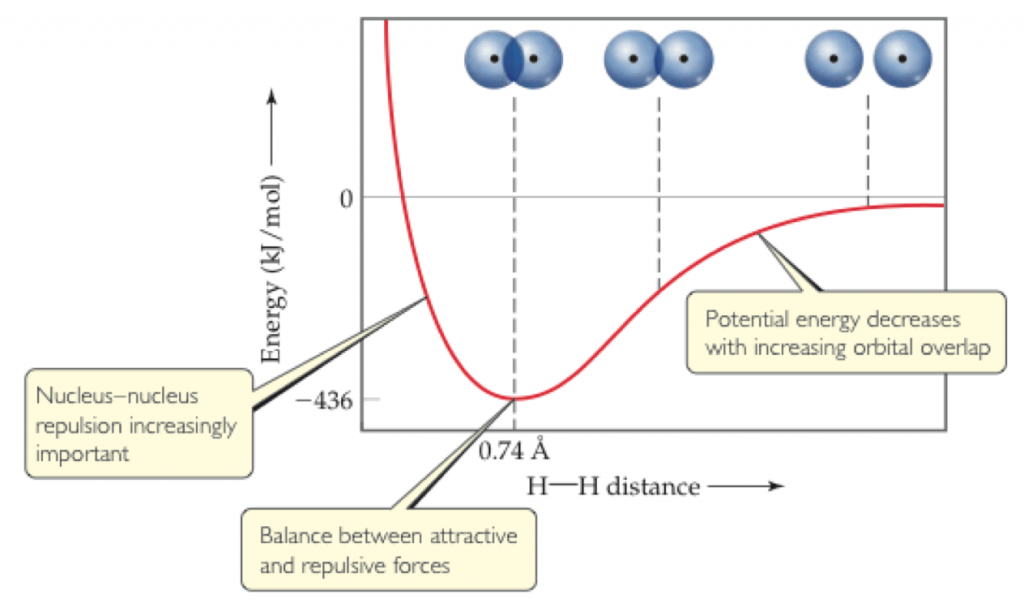
But don’t worry if you didn’t get the physical explanation! The concept of steric effects is actually much more simple than that!
It boils down to this: It will be much easier for a nucleophile to approach an electrophilic center if the latter is more accessible (which means, if it has less bulky substituents around it).
How Do Steric Effects Affect Chemical Reactions?
The following example extracted from Wade’s Organic Chemistry is perfect for visualizing this basic organic chemistry concept:
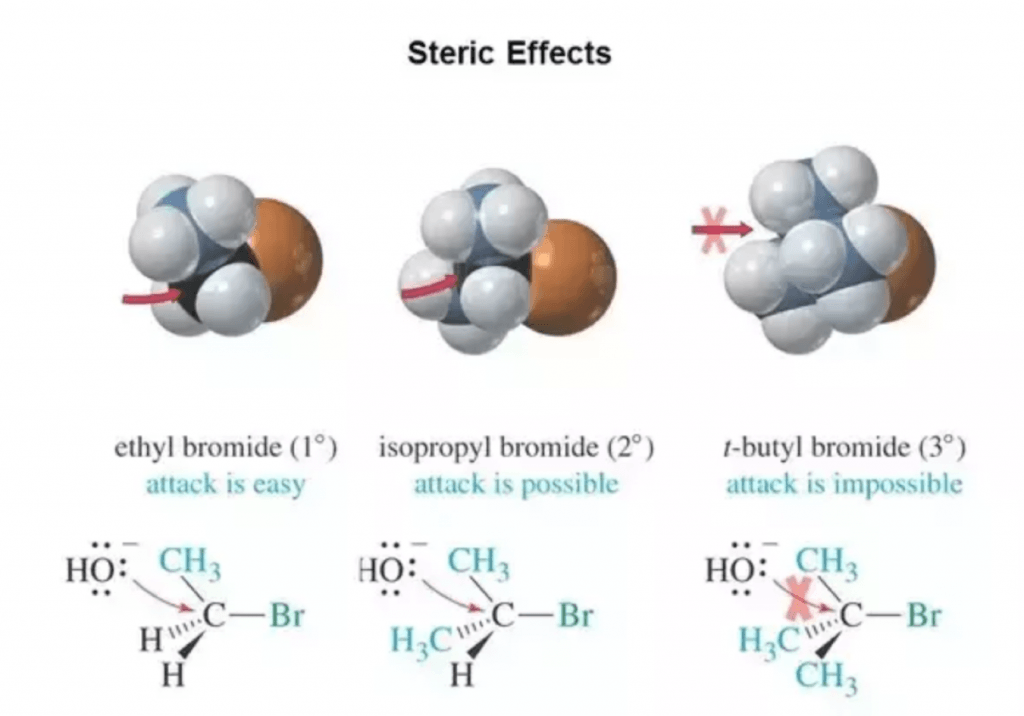
The SN2 reaction will happen very fast in ethyl bromide (left). The electrophilic carbon is very accessible, and the reaction will take place smoothly.
On the other hand, isopropyl bromide, has a secondary electrophilic carbon. This center will be much more shielded (it has a bulkier environment around it), so a nuclophilic substitution reaction can take place, but it will be much slower than for the primary one. This is due to the higher repulsive interactions that will exist between the nucleophilic molecule (in this case, OH–) and the two methyl groups of the electrophilic molecule.
In the third case, we have a tertiary alkyl bromide. The electrophilic carbon will be completely blocked by the tert-butyl group. The electrophile cannot approach the electrophilic center, and the SN2 reaction will never take place on this substrate.
However, there are other mechanisms (SN1) that may allow this reaction to proceed in some cases. But it will definitely never take place through a penta-coordinated transition of state typical of concerted SN2 reactions.
We have covered this in another tutorial review about SN2 and SN1 reactions, and how do they link to steric effects.
A Real Example of Steric Effects in Basic Organic Chemistry
We can go further into the basic organic chemistry concept of steric effects with a real life example.
Look at the Finkelstein reaction of the dibromoalkane below:
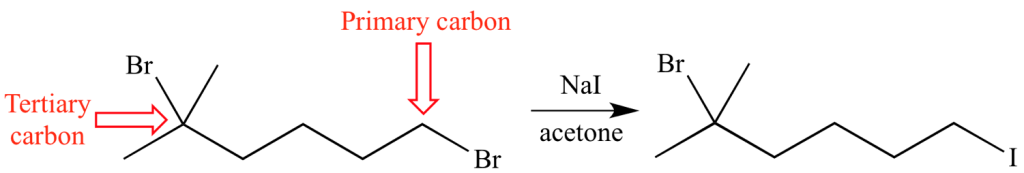
This reaction goes through a simple concerted (this means that the nucleophile -iodide- attacks at the same time than the leaving group -bromide- is leaving the molecule) nucleophilic attack of I– from the sodium iodide to the alkyl bromide.
There are two electrophilic carbons attached to a bromide that can in principle react. However, only the product of substitution of the primary one is obtained! The tertiary bromide is intact.
These are steric effects in action.
Effect of Distal Bulky Groups in Organic Reactions
Steric hindrance can also play a role if the physically big substituents are in carbon atoms that are not actually reacting. Neighboring substituents with high volume can define the speed of a chemical reaction.
An example is a simple hydrolysis of an ester.
The bigger the substituents found nearby, the less accessible the reactive electrophilic center will be, and as a result, the hydrolysis will be slower:
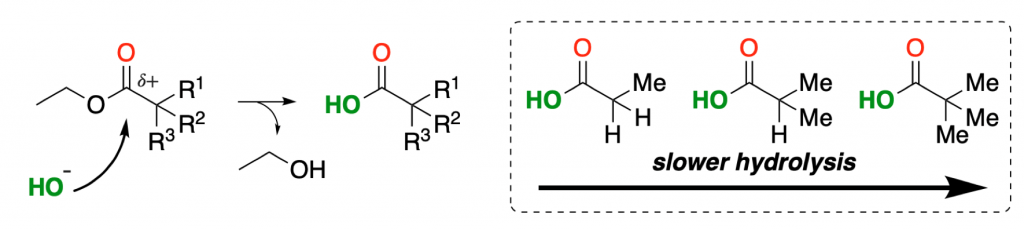
Steric effects can be easily visualized by using a molecular model kit. We have previously discussed how to use them for that and other purposes.
This brings us to the endgame of this concept review: merging together electronic and steric effects.
How Do We Combine Electronic and Steric Effects in a Single Concept?
Now that we know perfectly what both electronic effects and steric effects are, understanding situation in which both of them play a role at the same time will be fairly straightforward!
But the reactivity of a molecule can be controlled by both effects at the same time.
Now, this can get as complicated or complex as you want, but very simple reactions such as electrophilic aromatic substitution (EAS) can be used as examples.
Introducing Electrophilic Aromatic Substitutions
We are not going to review this classical reaction from scratch, it would be too lengthy for the purpose of this article. There is plenty of material about this transformation in other sites.
But we will obviously review the basics. The most typical example of EAS is the nitration of an aromatic compound by treatment with HNO3 and H2SO4.
The benzene ring acts as nucleophile attacking the NO2+ cation, an electrophile generated in situ.
The reaction goes through a positively-charged Wheland intermediate, which then gets rearomatized by abstraction of a proton (H+). This gives the nitrated aromatic ring.
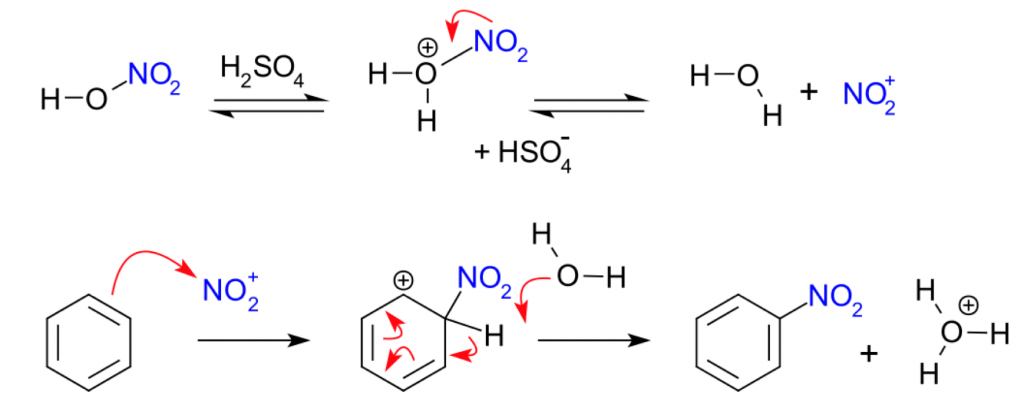
The interesting part is when, instead of plain benzene, we use substituted benzenes as starting materials.
Electronic Effects in EAS: A Basic Organic Chemistry Concept
The substituents in the aromatic ring define its reactivity towards electrophilic aromatic substitution.
As we mentioned, the aromatic ring acts as a nucleophile. Therefore, it will be much more reactive if it has substituents that donate electrons to the ring. This increases the electron density on the delocalized aromatic bonds, making it a better nucleophile.
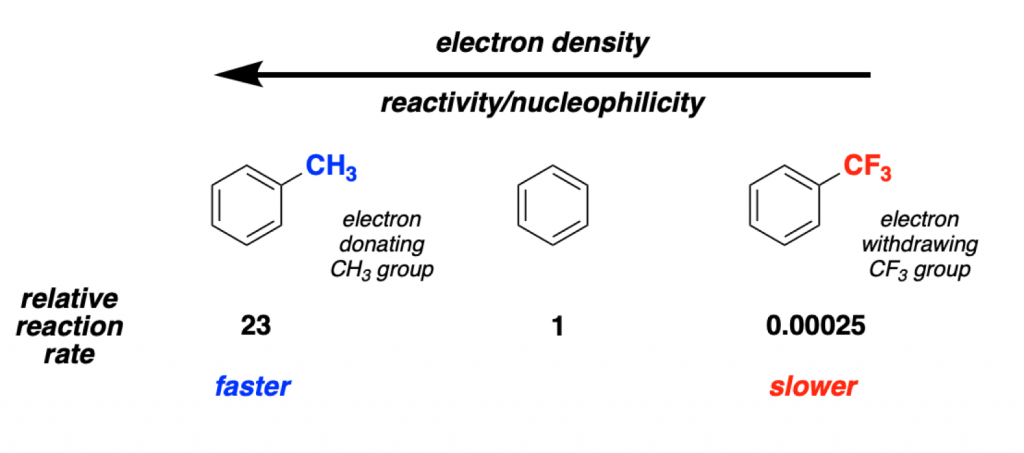
As you can see in the picture, putting an electron donating group (such as a Me, MeO or NMe2) in the ring, it will get activated. The ring will have a larger density of electrons, and be more nucleophilic (in effect, more reactive).
On the other hand, if you add in an electron withdrawing group (such as CF3, CO2R or NO2), the effect will be the opposite. The ring gets poorer in electrons, and therefore, deactivated.
Adding in Steric Effects to Basic Organic Chemistry
The nature of the substituents affects more factors, such as the regioselectivity of the reaction (in which position will the electrophilic substitution occur). This site has plenty of information about that.
But for the purpose of this post, it is worth remarking that, alkyl groups such as methyl, are activating groups (via induction) that also direct EAS towards the ortho and para positions of the ring.
You probably already guess where this is going…
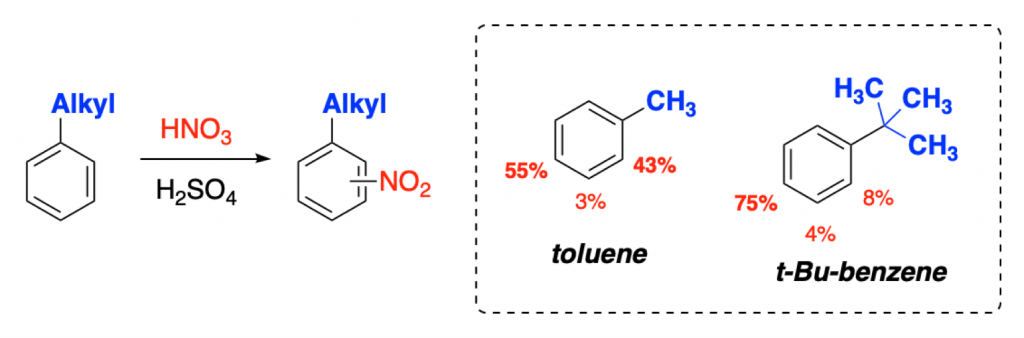
Well, if you take toluene (methylbenzene), you get, as expected, an almost 50:50 mixture of ortho and para substituted products, in a rate around 20 times faster than for naked benzene.
On the other hand, if you take tert-butyl benzene, you get much higher selectivity. Although electronically both Me and tBu groups are almost the same, tBu is a much bigger substituent. Steric effects come into play!
With a bigger alkyl group, the ratio is not an ortho/para almost statistical (1:1) distribution. Instead, you get a 75:8 ratio, favoring the para substituted one.
Why? Because the tert-butyl group is so bulky that, due to steric effects, given the choice between the two electronically-favored positions (ortho and para), the electrophile will approach the ring through the one that is further away (less repulsion) from the big group (the para position).
This is a great example of merging together electronic effects and steric effects. As you can see, the answer to our original question of “what is the most important basic organic chemistry concept” is clear. It is actually not a single concept, but a combination of these two.
I hope you have enjoyed these organic chemistry definitions and examples. Be sure to check out our tutorial on why do chemicals react, according to thermodynamics and kinetics.
Now it’s the time for you to participate!
Head on to the comment section and post any suggestion, question or another examples you might find interesting about these concepts.
Besides, feel free to share this content with your students or colleagues, or in your website, I hope it can help the most people possible!

I don’t understand what all the “me”s stand for I think it’s distracting. Please elaborate.