A universal challenge that has been keeping chemists busy ever since the modern concepts of “atom” and “molecules” were conceived, is how to actually characterize molecular compounds, either human-made or found on Nature. But, can we see real atoms and molecules?
Confirming the actual structure of a molecule, is still a big challenge now-a-days. The advances in techniques such as NMR (Nuclear Magnetic Resonance) spectroscopy, or single-crystal X-ray diffraction have significantly helped speeding up this problem.
Molecular structure determination
Every month we get reports of chemical structures whose structures have to be reassigned or revised after some study (either synthetic or just based on characterization techniques) is carried out. On this regard, it is worth remarking the difference between scientific models and reality.
Truth is, even today, the methods for the characterization of molecules available to use routinely (which are explained in the most basic chemical bibliography), can be consider rather rudimentary, and of difficult interpretation for non-experts. Let me be honest, I am a trained PhD organic chemist and if I had to take a look at the NMR spectra of a complex natural product such as maitotoxin, I would probably have no clue what I am looking at.
Single crystal X-ray diffraction is probably the closest method to easily visualize the structure of a molecule in 3D. However, this is not a bulletproof method. The sample preparation (growing single crystals) required for this indirect technique, renders it useless for a wide variety of chemical compounds.
Can we actually see real molecules or atoms?
Accordingly, I would say that by today, there should already be a method that allows taking a direct microscopic “picture” of any compound you like, and immediately visualizing its structure in a screen. Apparently we are not quite there yet (in regards to “any compound”, keep reading). However, the answer may come under the name of atomic microscopy, and all of its variations.
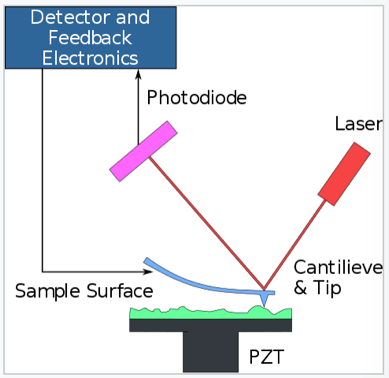
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very high resolution probe-microscopy technique. It allows us to actually “see” or “take real pictures” at the nanometer-scale, in which the molecular realm lies. A picture is worth 1000 words. In the example below, scientists make use of this technique to get pictures of a compound called naphthalenetetracarboxylic diimide. We can actually see a real molecule.
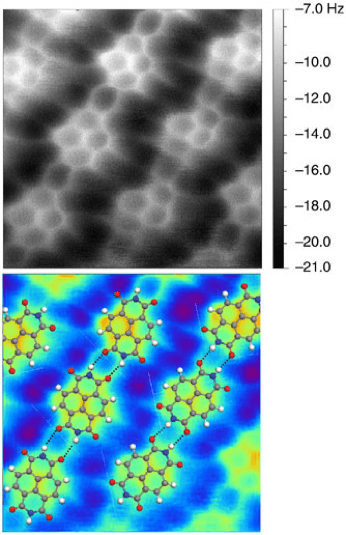
Much more recently, researchers at Oxford and IBM used STM-AFM to generate and visualize in situ the first cyclic allotrope of carbon, cyclo[18]carbon.
Seeing atoms in motion
The world of visualizing at the atomic level took a leap more than ten years ago. In 2008, a research group reported the imaging for the first time of light atoms and molecules on graphene. Subsequently, the same team managed to observe for the first time the actual movement of insolated graphene atoms in real time. The following movie from the Berkeley team shows the growth of a hole in a graphene sheet. For this experiment, a beam of electrons is focused to a specific spot on the graphene sheet, blowing out the focused carbon atoms making a hole. Besides, it can also be observed how the carbon atoms rearrange themselves (edge reconstruction) to adapt a more stable configuration.
The Boy And His Atom: The World’s Smallest Movie
The Guinness World Record for the “Smallest Stop-Motion Film” is held by a movie recorded by IBM scientists. Sometimes, nanophysicists also need to have a bit of fun, and what they decided is to “film” a movie by using scanning tunneling microscopy, a the result is in the following video:
By the use of this technique, the scientists managed to move a lot of molecules of carbon dioxide following their will. The result is a movie you can only see using a microscope that magnifies one hundred million times.
Direct observation of chemical reactions
Obviously, taking real pictures of molecules and atoms was just not enough for the scientific community. If we fast-forward to year 2013, atomic microscopy, more specifically, non-contact atomic force microscopy, allowed the direct imaging of molecular structures during a chemical reaction. Some results of these experiments published in the journal Science are displayed below. We cannot only see actual atoms molecules, we can observe directly chemical reactions!
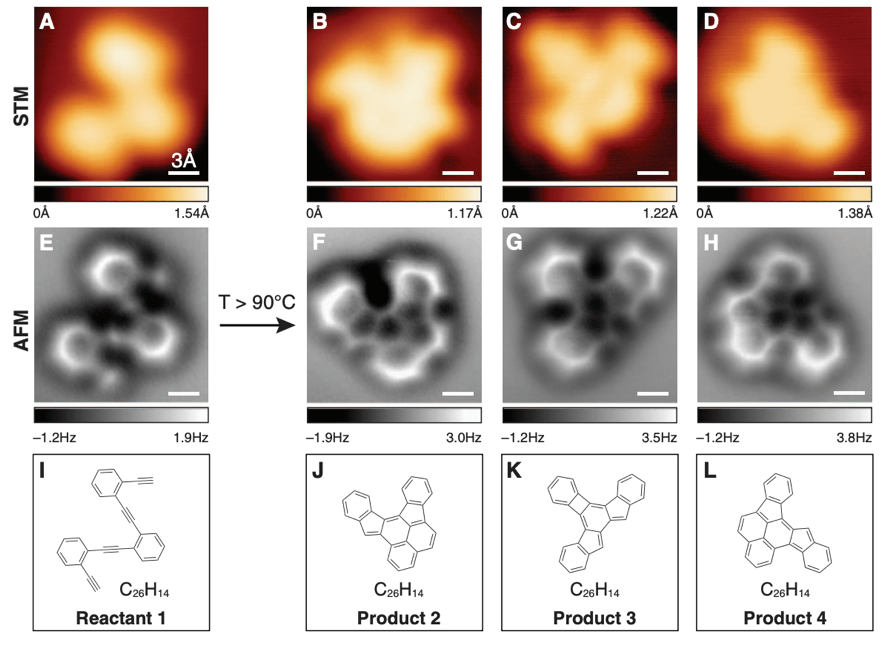
AFM in structural determination
This field started as a cluster of isolated cases, but as the years went by, more and more examples of the application of this set of physical techniques are being constantly reported. The level at which the studied molecules can be observed is rather impressive. A recent example is the actual structural determination of a natural compound, breitfussin A. Several functional groups of the molecule were derived from classical spectroscopic data (a). Then, an AFM image (c) allowed observing the real structure of the molecule, placing each piece of the puzzle (a) in the correct spot. This established the previously unknown structure of the molecule (b).
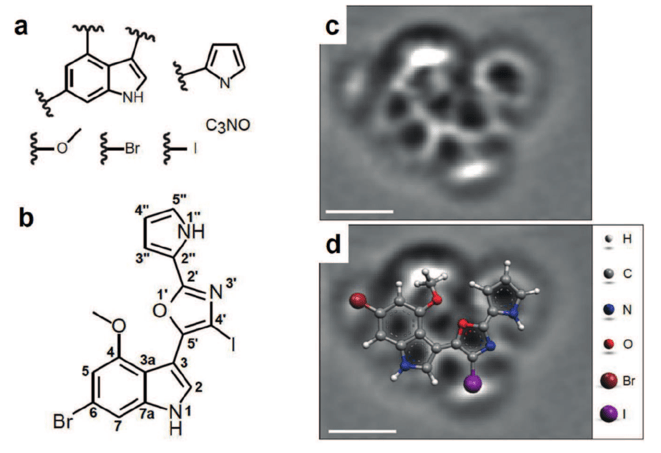
Taking real pictures of complex chemical reactions
On the reactivity side of things, much more recently, it was possible to directly image the course of a reaction called the Bergman cyclization. This is one of the most fascinating rearrangements in chemistry. The chemical transformation is directly induced in the metal surface in which the atomic microscopy procedure is carried out.
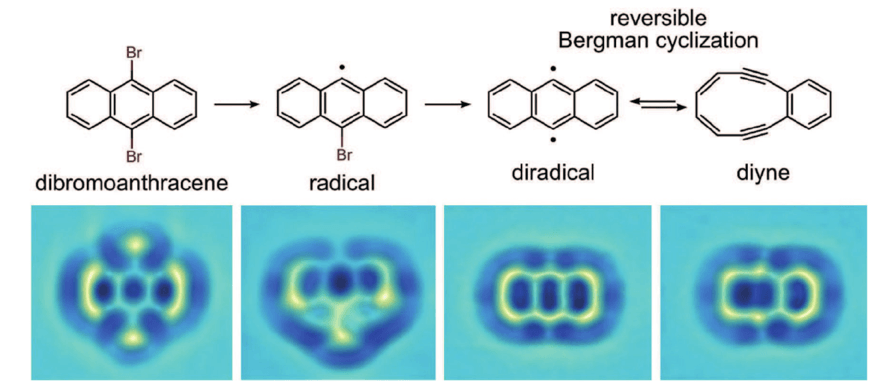
However, as stated at the end of the introduction, not every molecule or reaction can be a candidate for a STM study as these. Several conditions need to be met. One of them (which might have already called your attention) is that the analyzed compounds need to be near-planar. These techniques rely on depositing the molecules of the compound in a planar metal surface, so planar molecules are the ones that give more interpretable data.
The search for the “Holy Grail” of structural determination
To finish this short essay that does not make justice to the whole field of molecular imaging, a recent application of what is called micro-electron diffraction (MicroED) will be discussed. This brilliant application of electron diffraction, allows overcoming probably the biggest problem on classical X-ray diffraction methods: the requirement of crystalline material of the molecule which structure wants to be elucidated.
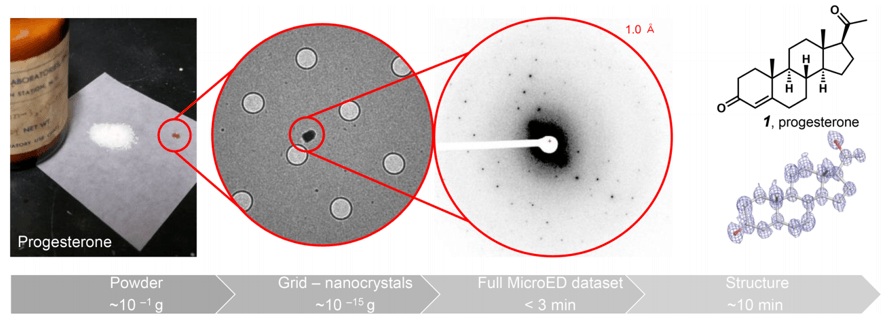
This technique allows taking simple powder of any non-crystalline solid, without almost any sample preparation, and getting 3D structures of the powder nano-crystals in a matter of minutes, with extremely high resolutions. The structure of molecules with very high complexity, as thiostrepton, could be obtained unequivocally.
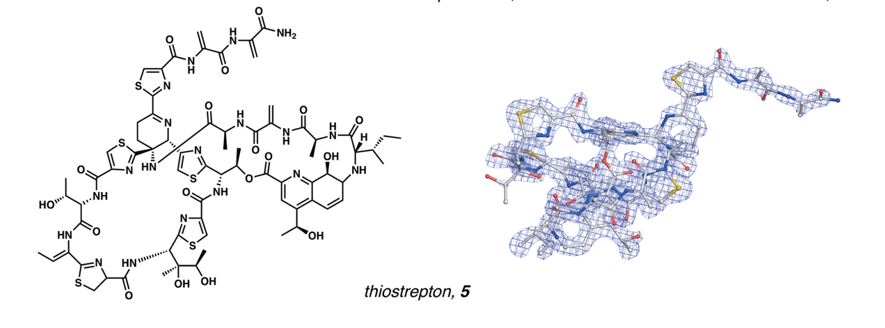
Is this the future of chemistry?
Can we see real atoms and molecules at this point? I would say that we definitely can. All the results that have been described in this article were published only over the last decade. Atomic microscopy seems to be here to stay, and it might be one of the tools that finally allows chemists to stop relying in rudimentary techniques for the determination of molecular structures. Only time will tell.
Stay tuned for more posts about the future of chemistry, share, and post your thoughts in the comment section!

Awesome article.
I’m confused still. Not a scientist but curious. Can we see an atom? Like we can see an object with our eyes but with the use of microscope? Not by inference but actually see the atom.