There are many allotropes of carbon. Allotropes are forms of the same element which have different structures. But today, the synthesis of the first cyclic carbon allotrope was reported in Science.
Some of the most well-known forms of carbon are diamond (a) or graphite (b). Other allotropes are fullerenes (d, e, f), amorphous carbon (g) or carbon nanotubes (h).

But until today, it was not possible to characterize a cyclic molecular all-carbon ring. Researchers at University of Oxford teamed up with scientists at IBM, and resurrected this long-forgotten challenge.
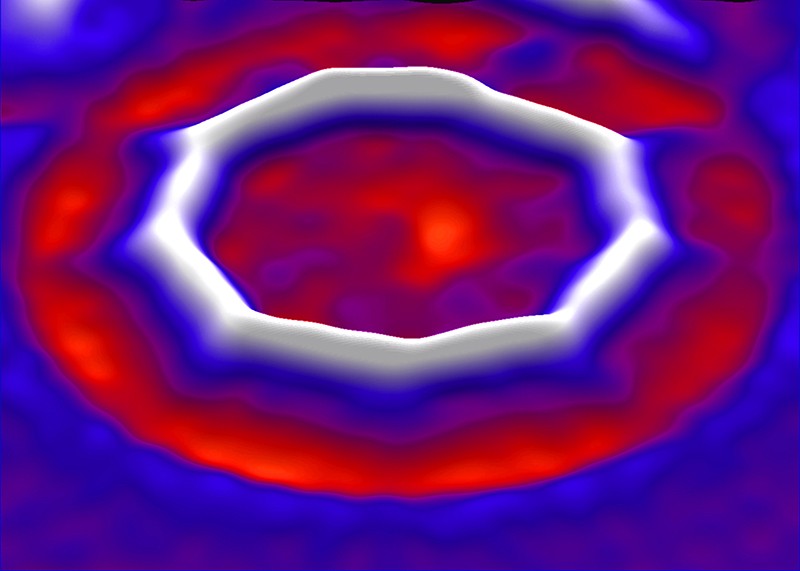
The result is spectacular: a circle of 18 sp-hybridized carbon atoms, held together by alternating single and triple bonds.
The challenge of building such molecule is actually not new. In fact, the basis for this recent work were already established in 1990.
Initial Work Towards Cyclocarbon
At that time, while pursuing the synthesis of the same kind of cyclic carbon allotrope, cyclo[18]carbon, the group of François Diederich reported in the Journal of the American Chemical Society the synthesis of and characterization of a stable hexacobalt complex of this cyclic material.
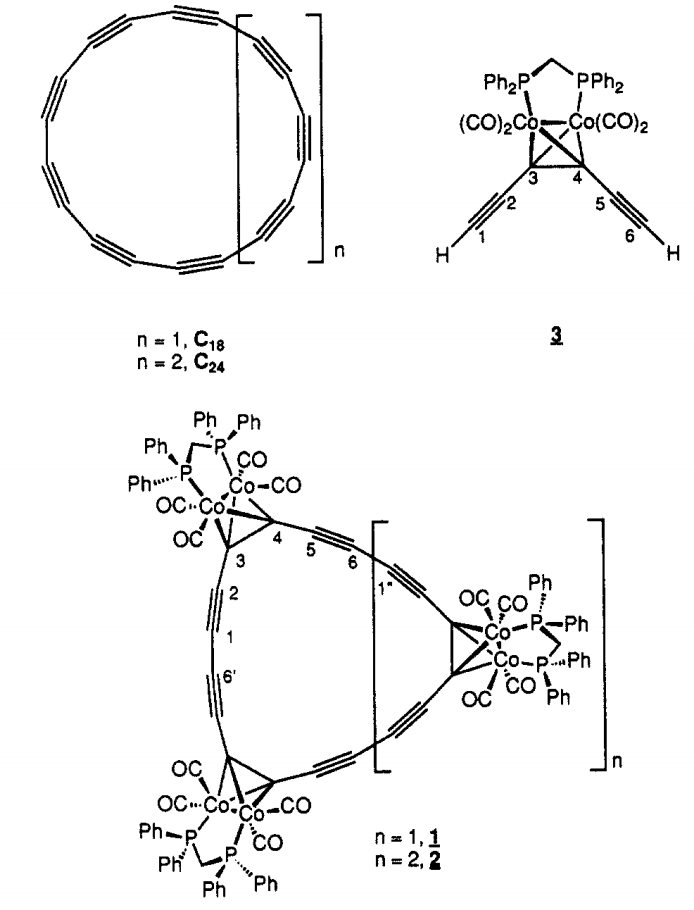
This structurally peculiar coordination complex may seem to be really close to the desired all-carbon cyclic molecule, but this couldn’t be further from the truth.
In fact, almost 30 years passed since Diederich and his team made the initial steps towards this target, until today.
Synthesis and Characterization of the First Cyclic Carbon Allotrope
Fast-forward to 2019, P. Gawel, H. L. Anderson (Oxford University), L. Gross (IBM) and co-workers finally completed this challenge.
How did they do it? Basically they made a great use of Scanning Tunneling Microscopy (STM).
STM is a technique based in quantum tunneling. In STM, you usually deposit the molecule you want to study in a metal surface. Then a voltage is applied through a very small conducting tip, which allows electrons to “tunnel”, generating a current. Analyzing changes in the system, you can extract information and display it as images.
Thanks to the most recent advances in STM and atomic force microscopy (ATM), it is possible to obtain and see images of molecules with great resoluion.
Diederich group had developed the synthesis of several cyclocarbon oxides (shown as precursors in the pictures below).
Cyclocarbon oxide (C24O6), a triangular oxygenated molecule, was deposited on a bilayer of NaCl on a Cu(111) surface. Then it was submitted to low-temperature STM-AFM.
Under these conditions, the starting molecule loses CO as carbon monoxide. This process can occur multiple times, until the all-carbon 18-membered ring, cyclocarbon, is obtained. The molecule can be imaged in-situ (and therefore, characterized) at the same time. In this manner, the first cyclic allotrope of carbon was finally prepared.
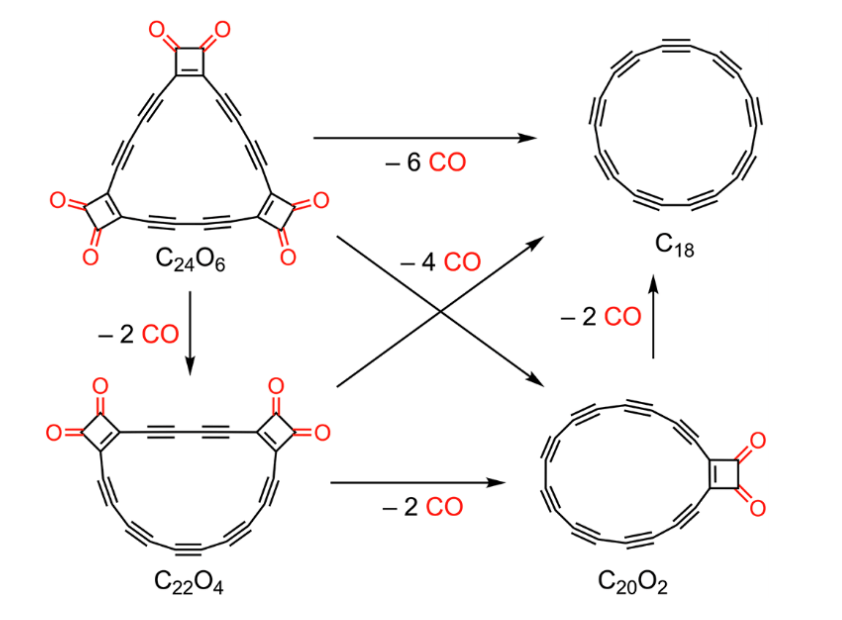
The decarbonylation process takes place step by step (one molecule of CO at a time). This allows not only to see images of the final cyclocarbon, but also of several decarbonylated intermediates.
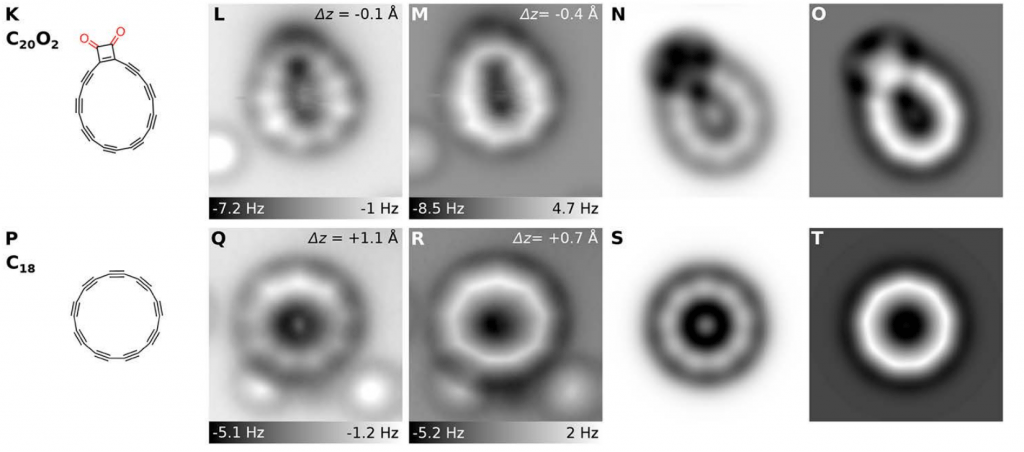
It is hard to tell if the resulting structure is “aromatic”, since classical Hückel rules cannot be applied to such complex systems. In fact, one could argue that this product is borderline between inorganic and organic chemistry.
Many scientist had tried before to make and observe this elusive structure. But why?
The Significance of the First Cyclic Carbon Allotrope
Well, for starters, initial studies on cyclo[18]carbon suggest that this new material can act as a semiconductor, which is the main appeal for the synthesis of new carbon allotropes, or other compounds such as polyaromatic hydrocarbons.
From a purely academic point of view, this specific structure is significantly less stable than any other of the well-known carbon allotropes. Therefore, it was a significant scientific challenge to overcome. It took 30 years and mastering modern techniques such as STM-AFM to actually see a molecule that many didn’t think we would see any time soon.
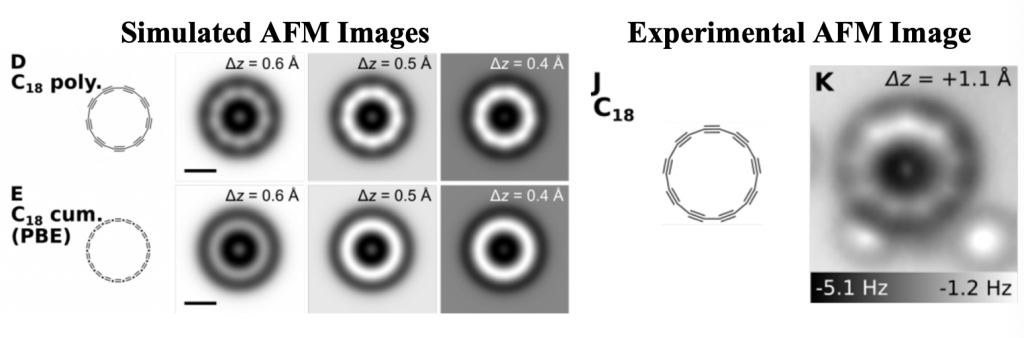
This discovery also allowed scientist to solve an old mistery. Are cyclic all-carbon molecules polyynic (alternating double and triple bonds) or cummulenic (consecutive double bonds)?
As you can see in the image below, the experimental image of the cyclic allotrope of carbon, perfectly matches with a predicted polyyne, made of alternating triple and single bonds.
So far this is very fundamental research, since these techniques only allow making “one molecule at a time”, and at under very specific conditions. This doesn’t allow actually preparing enough amount of material to test potential applications. However, this is a big leap forward in nanomaterials chemistry, and for sure this discovery will lead the scientific community to many relevant findings.
All credit to:
An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. K. Kaiser, L. M. Scriven, F. Schulz, P. Gawel, L. Gross, H. L. Anderson Science 2019, doi: 10.1126/science.aay1914

Yeah, what’s up with fullerenes these days? I worked under Harry Dorn an Don Bethune. And I certainly remembered Rick Smally. My area was in endohedrials with the Lanthanide group. I worked in the early 1990s on purifing them. So , have they gone anywhere? Are they used for any practical application: Superconductor apps, nonlinear optocs, delivery of cancer drugs?