Ever wondered how to apply your chemistry knowledge on how to make your own soap, understanding the process?
Making soap at home is a fun and crafty way to get some firsthand chemistry experience and enjoy the fruits of your labor.
Soap and soapmaking encompass many chemistry concepts:
- Acids, Bases and Salts
- Biochemistry: Lipids
- Organic Chemistry: Esters, Carboxylic Acids and Alcohols
- Hydrolysis
- Emulsification
- Hydrophilic/Hydrophobic Interactions
But don’t be intimidated—the actual process of making homemade soap is easier than you think!
Read on for a summary of the chemistry behind soapmaking, the chemical reaction that is used to make soap, and the science of how soap works. Most of these concepts are further expanded in any organic chemistry textbooks, which we have reviewed here.
Otherwise, skip to the bottom of the post for a simple beginner-friendly DIY soap recipe.
It is a fun experiment with kids, but always supervised! If you want to do chemistry with children, make sure to get one of the best chemistry sets for kids and adults out there!
The Chemistry of Soapmaking: Saponification
The chemical reaction that produces soap is so ancient and characteristic that its name literally means “to turn into soap”.
Saponification, from sapo, the Latin word for soap, is one of the more memorable chemical reactions learned in the first semester of organic chemistry because of its obvious applications in everyday life.
Here is a Summary of the Overall Reaction
First, we begin with a triglyceride (the fatty molecules found in vegetable oils and animal fats). A strong base is added, which breaks the ester bonds of the triglyceride into three carboxylic acids and glyceroxide. Finally, after proton exchange, the products are three carboxylic acid salts and glycerol.
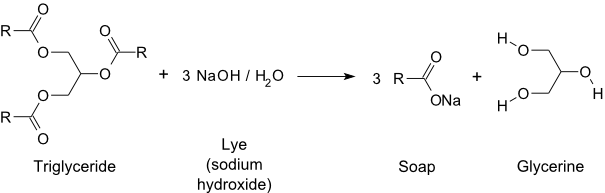
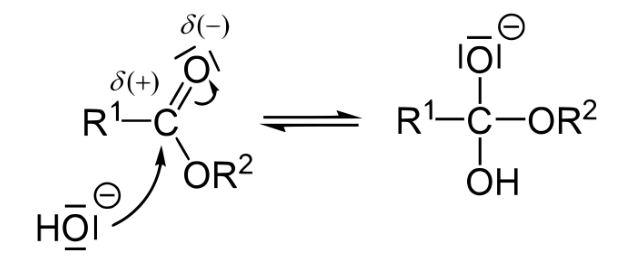
Explanation of the Saponification Reaction Mechanism
Saponification is an alkaline hydrolysis of an ester. You may recall that the formation of an ester is a dehydration reaction between a carboxylic acid and an alcohol.
In biochemistry, this reaction creates a triglyceride from three free fatty acid chains and one molecule of glycerol. Saponification uses a strong base to essentially undo that reaction. We have explored this and other simple reactions in this tutorial review about organic chemistry concepts.
Remember that a carbonyl carbon, such as the one in an ester bond, has a partial positive charge due to both resonance and the greater electronegativity of the bonded oxygen. Because of this, it is a good target of nucleophilic attack by the hydroxide ion. The product of this step is an orthoester intermediate (note the negative charge on the former carboxyl oxygen).
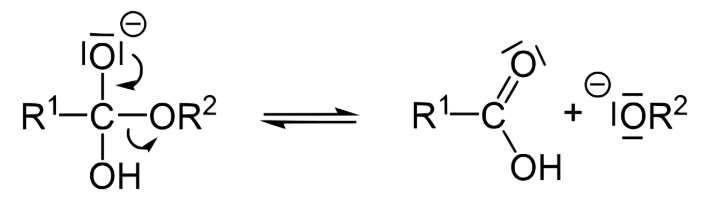
Remember that oxygen is most stable when it has two bonds and two lone pairs of electrons. Its electrons rearrange in order to achieve this stability, reforming the double bond with carbon to make a carboxylic acid and expelling the other half of the ester as an alkoxide, in a second step called “elimination” (the conjugate base of an alcohol).
We know that alcohols in general are very weak acids. Their conjugate bases, alkoxides, are therefore quite strong. As a result, proton exchange takes place, and the acidic proton of the carboxylic acid is readily donated to the alkoxide. This results on the formation of an alcohol, plus the sodium or potassium salt of the carboxylic acid.
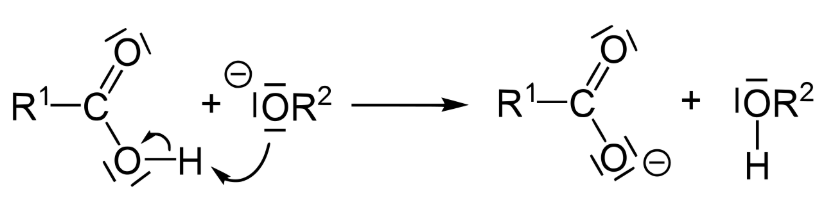
Products of Saponification
This same reaction is occurring at all three ester bonds in a triglyceride during saponification. The three resulting fatty acid salts are also known as soap salts. Their properties are highly dependent on the number of carbons in the fatty acid chain and the degree of saturation.
Longer carbon chains (stearic acid, C18, for instance) tend to yield soaps that are harder and less soluble.
On the other hand, unsaturated fatty acids will yield a softer soap with a lower melting point. Some fatty acid salts are more cleansing than conditioning, and vice versa. Similarly, some will work up a nice, rich lather, while others will not. It’s important to consider these effects when deciding which fats and oils to use in a soap recipe.
How does soap work?
Take a look at the molecular structure of this soap salt:
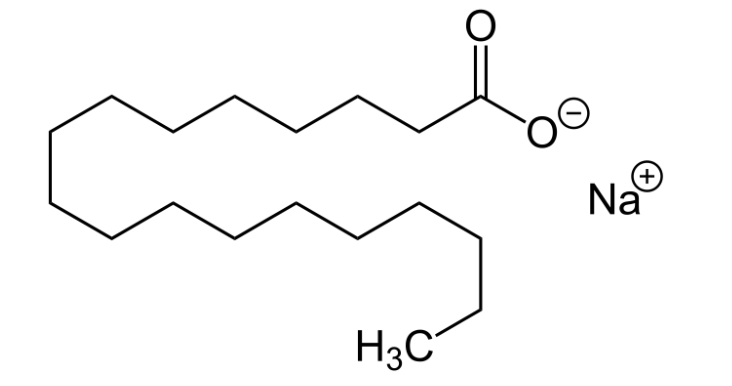
Is this molecule polar, or is it non-polar? The answer is, both! At the top right, the carboxylate, acting as the anion in this sodium salt, is a very polar functional group. However, the rest of that long hydrocarbon chain is non-polar.
This type of compounds are called amphipathic.
Everyone knows that oil and water don’t mix, but not everyone knows that the reason for this is the polarity (or lack thereof) of each substance. As a chemistry student, you have probably already learned that like dissolves like, and that polar molecules are hydrophilic (water-loving) while nonpolar molecules are hydrophobic (water-fearing).
You can probably see where this is going… Soap works by allowing hydrophobic substances, like grease and oil, to dissolve in water. Its two ends, one polar and the other nonpolar, allow it to mix with both water and oil. It does this by forming tiny spherical structures called micelles.
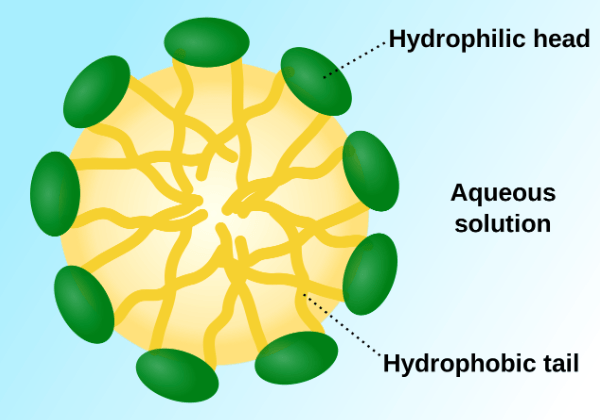
In this cross-section of a micelle, you can see the hydrophobic (nonpolar) hydrocarbon chains inside the sphere, while the hydrophilic (polar) ends form the surface. When you wash a greasy pan with soapy water, the grease is attracted to the hydrophobic ends, trapping it inside these micelles. Since the surface of the micelle is polar, it is soluble in water and can now be easily rinsed away.
Soap is also a natural surfactant, which means it reduces the surface tension of water, effectively making water “wetter”. The “wetter” the water is, the better its solvent properties are, and the better it cleans.
Chemistry at Home: How to Make Your Own Soap
Now, let’s put all this chemistry knowledge into action and make some homemade soap!
This DIY (do it yourself) soap recipe is a very basic one that is perfect for beginners. You will need some basic equipment and a few easily accessible ingredients:
Soapmaking Equipment
- Safety goggles and gloves
- Kitchen scale
- Pitcher*
- Jar*
- Large pot or bowl*
- Thermometer
- Mixing spoons*
- Immersion blender (stick blender)
- Rubber spatula
- Soap molds
* Make sure you use nonreactive materials, like plastic or glass.
You can find our personal recommendations on home chemistry labware here. It would be ideal to be able to use appropriate beakers or flasks for this experiments. But if you don’t have access to those yet, you can get away with the household items listed above.
Lye is caustic (very basic) and can react with many things, as metals (or you skin!). This reaction is exothermic, and the rapid temperature change may cause low-quality glass containers to crack.
Basic Soap Ingredients:
- 500 g of olive oil
- 100 g of coconut oil
- 80 g of lye (NaOH, caustic soda)
- 200 mL of water
Keep in mind that, while you can use practically any type of oil or fat to make soap, they will have vastly different properties and may require different amounts of lye. If you use different oils, make sure you use a lye calculator to ensure you aren’t using too much. Having excess oil in your soap is not a big deal, but having excess lye is!
Instructions:
- Prepare the lye/NaOH solution. Weigh the water in the pitcher. Separately, weigh the NaOH into the jar. Then, slowly add the NaOH to the water. DO NOT ADD THE WATER TO THE LYE! Remember how they made you memorize “add acid to water, not water to acid” in your chemistry lab’s safety module? The same goes for bases. A strong base like NaOH is highly reactive by definition. That means a LOT of energy is released when it makes contact with water. If you do this step backwards, the mixture will quickly heat up. This might end-up in a caustic soda geyser, which can easily cause a chemical burn. When you add NaOH to water, there will still be heat release, but it will be much less dangerous. Carefully stir to dissolve (rinse the spoon immediately after mixing). This solution can get to almost boiling temperature all by itself, so you may need to allow it to cool down slightly until you can easily handle the pitcher.
- Weigh out and mix your oils in the pot or bowl. This will be easier if you warm the coconut oil up a bit first until it melts.
- Carefully add the NaOH solution to the oil mixture and gently mix with a spoon until it gives an homogeneous mixture.
- Now you can pull out your stick blender and start the emulsifying process. Remember, you are blending a highly caustic mixture right now, so keep your distance and try not to splatter.
- After a few minutes of blending, the mixture should start to thicken, indicating that the saponification reaction is underway. Optionally, this is when you can add other ingredients to customize your soap, like essential oils, colorants, mix-ins, etc. Otherwise, you can proceed with transferring the soap to whatever you are using as molds. Silicone baking molds work great for this. Use a rubber spatula to get every last bit out and facilitate cleanup.
- Let your soap harden for at least 24 hours before trying to remove it from the mold. Once it is hard enough to handle, you can cut it into different shapes/sizes if desired.
- Finally, the soap needs to cure for about one month to be sure the saponification reaction is complete and to dry out the excess water. Once it’s fully cured, you can enjoy using your homemade soap!
And that is pretty much it! As you can see, it is an easy procedure.
Now that you know how to make your own soap, and you understand the chemistry behind it, time to put the experimental procedure into practice!
Let us know in the comments if you have any question or suggestion. Also, feel free to share the results of your first batch of soap!

I’ve made the soap, but I cannot get the fragrance from essential oils to stay. Do you have any suggestions?
Thank you.
Hey Nora,
Which kind of fragances are you using?
I actually never used any fragances myself, but you can find some info on that here. You need to use fragances that can resist the lye treatment, that can be pretty harsh for some materials. If the molecules responsible for the smell react with anhy of your components, you will most likely lose the smell.
Thank you for this important explanation. But i do have one request: Would you, please, send me the effect of each recipe on the soap property in terms of chemistry?