Chemistry is fascinating, I’m sure we all agree on that. Not only that: thanks to chemistry we know of many compounds that are extremely harmful if not handled properly. Today we will share with you a comprehensive list of the most dangerous chemicals known to man.
Chemicals are not bad (even not-organic ones). The thing is, everything we see or touch in our world, is made up of chemicals. From the purest distilled water that we use as solvent in the lab to a carrot that you just harvested from your backyard.
But there are many poisons out there in Nature, and other dangerous chemicals that may or may not be human-made.
On this informative article, we wanted to cover some of these scary compounds which you might want to steer away from.
If you are not a professional scientist, you probably will not encounter many of them in your every-day life, or for sure in any experiment that you might do at home but still is good, or interesting to be aware of them. or maybe you want to warn your chemist friend (together with a cool gift)
On the other hand, if you are a chemist, it is definitely possible that you might have to use some of these for your work. And it is always good to be prepared, so you can take the appropriate safety measurements. Or just nope the hell out of using them if ever asked to.
So, what is the most dangerous chemical known to man? What is the most toxic chemical?
What Kind of Dangerous Chemicals Are We Reviewing?
We have decided to divide the compounds in different categories.
This will depend on whether they are dangerous because they are poisonous (low LD50), corrosive, explosive, or extremely harmful chemicals for some reason.
We also have a category for typical dangerous laboratory chemicals (definitiely worth checking out if you’re a chemist or chemistry student).
Finally, we will also discuss some very dangerous compounds that can be found in every-day life situations.
In any case, all these nasty chemicals are interesting to know about.
Without further ado, let’s look into them!
The Most Dangerous Poisons
Botulinum toxin
Botulinum toxin, is basically the most lethal poison known to man. An average 70 kg human being only would have to take around 100 nanograms of this protein to die (it has an LD50 of 1.5–2.0 ng/kg).
If you put it in perspective, one gram of this toxin can kill more than one million people!
What this toxin does chemically is basically preventing acetylcholine from being released between neuron connexions. This breaks down the connexions of neurons with with muscle cells. This leads to muscle paralysis, as contraction of the muscle cells cannot take place.
If you take enough, the neuron connexions which make the heart or respiratory systems work go down, which can kill you.
Funnily enough, this toxin is used in medicine. Botulinum toxin is commercialized under the name of Botox, among others.
As a tool that can paralyze muscles, it found uses in treating muscle spasticity (muscles that are contracted all the time) or other muscle-related diseases. The most potent toxin in the world has even been used in cosmetics, in order to “smooth” facial muscles!
Batrachotoxin
Following next, the next poison comes off the skin of some dart frogs. Specifically, phyllobates terribilis or Golden Poison Frog is known for being one of the most dangerous poisonous animals in the wild.

One of the key components of their poison is batrachotoxin.
Contrary to botulinum toxin, which is a protein, batrachotoxin is a small organic molecule.
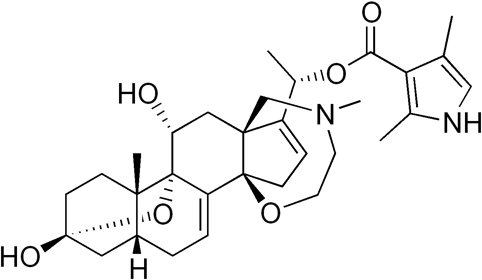
You can see how their structures have absolutely nothing to do with each other. But they both work in a fairly similar manner: batrachotoxin, with an LD50 of 2000 ng/kg (an order of magnitude less poisonous than botulinum toxin, but still scary), is also a neurotoxin. It blocks the Na+ ion channels permanently, preventing neurons from communicating with muscles, leading to paralysis and eventually heart failure.
Interestingly, dart frogs don’t make batrachotoxin by themselves.
They need to ingest certain alkaloids through their diet. If they are kept in captivity, dart frogs are rendered non-poisonous.
But in the wild, they make one of the most dangerous poison mixtures. Dart frogs can only be found in Colombia or Panama rain-forests, and they were used by indigenous tribes to make poisonous darts and arrows. That’s where they get their name from.
Ricin
We are back to the protein world with ricin. This is yet another order of magnitude less poisonous than botulinum toxin. Ricin’s LD50 is 22.000 ng/kg. But this still means that 2 mg of ricin will kill an average adult.

One of the most dangerous chemicals in the world, ricin, can be find in castor beans.
The mechanism of action of ricin is very different to the one for botulinum toxin or batrachotoxin.
This protein disrupts the ability of the body to assemble proteins from amino acids in the ribosomes.
Since the mechanism of action is much subtler than for other toxins, the symptoms take time to show up. But they eventually do. The inability to make proteins (a very basic type of cell metabolism, essential for cells to survive), causes damage to the nervous system, kidneys and liver in hours to several days.
Ricin got more popular around the world after its appearance in AMC show Breaking Bad.

Maitotoxin
We are coming back down in the LD50 score, since maitotoxin has a value of 50-130 ng/kg in mice. This is the highest for non-protein compounds. It is produced by a species of dinoflagellates, gambierdiscus toxicus, and can be found on the surface of some algae in Polynesia, or some animals such as the ciguateric fish.
But from a chemical point of view, the most interesting feature of maitotoxin is not its toxicity, but its chemical structure.
Maitotoxin is not a protein, but I wouldn’t call it a small molecule either. With a molecular weight of 3422 g/mol, maitotoxin is one of the toughest unbeaten synthetic challenges out there.
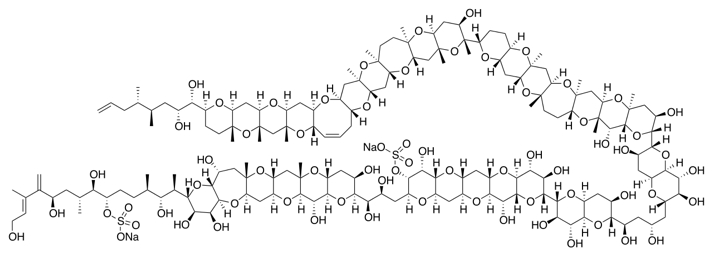
This impressive amphipathic structure made up of 32 fused rings and a handful of stereogenic centers has not been synthesized completely in organic chemistry labs. The research group of K. C. Nicolaou is involved on the total synthesis of this giant. So far, the synthesis of some of the ring domains has been published, but the entire molecule is still a challenge to overcome.
Tetrodotoxin
Tetrodotoxin is another small molecule, which similarly to batrachotoxin, is a potent neurotoxin. It is also a sodium channels blocker.
This poison is the one that makes dangerous several kinds of animals: fishes such as the porcupine fish or pufferfish. Also, blue-ringed octopuses or moon snails produce tetrodotoxin.
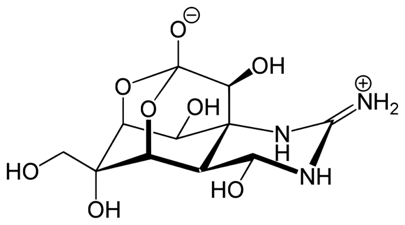
This is not a huge molecule as maitotoxin, but it is still an attractive synthetic target that has been the objective of many total synthesis project. The structure of the molecule was elucidated by Woodward in 1964, and the first total synthesis was reported in 1972.
Phosgene: COCl2
All the poisons covered above are made by natural sources. However, phosgene, a truly small molecule, is human-produced in the range of several tons a year.
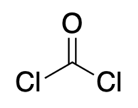
Phosgene, or carbonyl chloride, is classified as a chemical weapon, and it is responsible for many thousands of deaths during World Wars. A median concentration in air (LC50) of 200-500 parts per milion is enough to kill a person.

Despite being one of the most dangerous chemicals in history, it is an extremely useful reactive building block on chemical synthesis, and it is massively produced and used all over the world.
It is normally made by reaction carbon monoxide with chlorine gas, and it is employed in the synthesis of carbonates, isocyanates (precursors of polyurethanes), among others.
The Most Dangerous Acids and Bases
Hydrogen fluoride: HF
Hydrogen fluoride is not a very acidic acid. In fact, it’s the least acidic of all the hydrogen halides.
But it is actually the most dangerous.
HF is extremely toxic and corrosive. As it happens with many fluorine-containing compounds, it has weird properties.
Fluoride really loves binding to silicon, so HF can easily eat through glass (made of SiO2). This is the reason it needs to be handled and stored in plastic containers.
But HF can also bypass our skin barriers, and go through reaching the bones, dissolving them as CaF2 is formed.
The guys at Periodic Videos have performed some experiments showing how scary this acid can be:
As you can see, there is a difference between an acid being “strong” and being “corrosive”.
Fluoroantimonic acid: HSbF6
Fluoroantimonic acid is one of the most acidic compounds known to man. It is what is called a “superacid”. These are usually defined as chemicals which have an acidity (or ability to donate protons, H+) larger than pure sulfuric acid.
It is actually made by mixing HF with SbF5. Surprisingly, this combination between a relatively weak Brønsted acid (HF) and a Lewis acid (SbF5) gives rise to a compound which is 20.000.000.000.000.000.000 (2·1019) times stronger than H2SO4.
Piranha solution
Piranha solution is the name that chemists give to a mixture of hydrogen peroxide and sulfuric acid. H2SO4 and H2O2 react giving H2SO5 (Caro’s acid) and water.
This results on a strongly oxidizing acidic mixture. The “piranha” name is quite appropriate, since it easily eats through organic matter.
As a matter of fact, this mixture is used by chemists to remove organic residues from glassware (although the glassware has to be very valuable and all other common methods unsuccessful).
tert-Butyl lithium: t-BuLi
One of the most common dangerous laboratory chemicals is tert-butyl lithium. We have switched it to this category because it is an extremely strong base.
This makes it very useful in organic chemistry. If a proton cannot be abstracted by t-BuLi in an organic molecule, you will most likely not be able to remove it with anything else.
It can even react with THF (a common organic solvent, which usually are very chemically innert) at room temperature, removing one of their protons and leading to decomposition.

t-BuLi is very pyrophoric, it readily reacts with air catching fire, that’s why it has to be handled and stored with very special care, always under a protective inert atmosphere of pure nitrogen or argon.
The Most Dangerous Laboratory Chemicals
Dimethyl mercury: HgMe2
Karen Wetterhahn was a chemistry professor working on toxic metal exposures. Ironically, she died in 1997, due to exposure to dimethyl mercury.
One of the most famous dangerous lab chemicals is Me2Hg. Prof. Wetterhahn was using full protective equipment, but unfortunately, a couple of drops of dimethyl mercury fell in the top of her gloves. The amount of compound that could be absorbed through the gloves and her skin was enough to kill her by metal poisoning, slowly, after less than a year. Even using very strong chelation therapy, it was not possible to save her life.
Dimethyl mercury was used in very specialized NMR experiments using 199Hg nucleus, but I don’t think I would ever work with it. Me2Hg can actually go through most types of safety gloves. The use of this substance in any scenario is strongly discouraged.
The price of a potential accident is just too high.
Dimethyl cadmium: CdMe2
If you thought dimethyl mercury is nasty, meet its bigger brother, dimethyl cadmium. It is not only highly toxic as Me2Hg, but it is also highly reactive.
Dimethyl mercury reacts with air, or organic matter, not only exploding but also giving rise to more and more toxic Cd-compounds.
Dimethyl cadmium is also very volatile, and inhaling only a few micrograms of it can lead to cadmium metal poisoning, and eventually, to death.
Diazomethane: CH2N2
Diazomethane is the simplest diazo compound there is.
Diazo compounds are organic molecules which have a -N2 functional group attached to it. As you can imagine, thermodynamically, that nitrogen really wants to jump out of the organic molecule and leave as nitrogen gas.
So these compounds are extremely reactive, and some of them have a high tendency to explode. Besides, they are usually extremely toxic. Specifically, several deaths have been reported by diazomethane poisoning.
It is a useful reagent in organic chemistry, since nitrogen gas release is an extremely powerful driving force for achieving difficult chemical transformations, such as cyclopropanation or homologation reactions. Finding alternatives to diazomethane and its derivatives is an important challenge in modern organic chemistry.
Chloride trifluoride: ClF3
Chloride trifluoride is a compound which will look very unusual to you if you don’t have a very advanced chemistry knowledge. This is a hypervalent chlorine compound, with three fluorides attached to it.
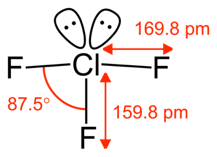
ClF3 is a very poisonous and reactive gas, which has found applications in fields such as rocket fuel research (although it is not used yet as rocket propellant), or as fuel in nuclear reactors.
It is manufactured by mixing F2 and Cl2 gases and then separating it by distillation.
It is a highly oxidizing agent. It can also act as a potent fluorinating compound.
Dioxygen difluoride: FOOF
As far as oxidants go, dioxygen difluoride is the top pick.
It can be prepared by mixing oxygen and fluorine gas, and the resulting compound is so oxidizing and unstable, that it starts decomposing even at temperatures as low as -160 ºC. It hass a funny structure, common to the one for classical peroxydes
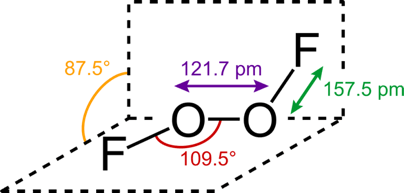
O2F2 reacts in a vigorous manner with virtually any chemical that it comes in contact with. It is usually referred to by the name “FOOF” due to its high tendency to make anything explode.
Osmium tetroxide: OsO4
Osmium tetroxide is not as scary as the last previous oxidants, but it is also a much more common laboratory chemical.
This reagent is great for oxidizing alkenes to diols, or for epoxydation reactions. As a matter of fact, most of the chemistry awarded one half of the 2001 Nobel prize in chemistry (to Barry Sharpless) is based on the use of osmium oxides for the asymmetric oxidation of alkenes.
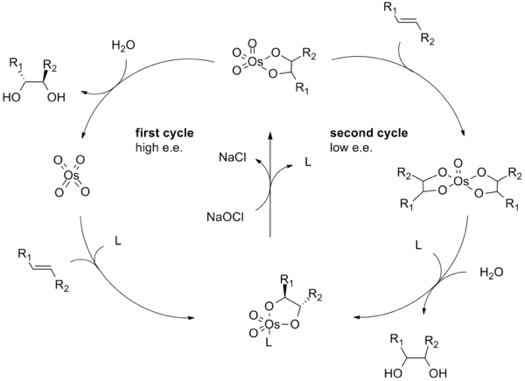
However, one should handle this chemical with care. OsO4 is very poisonous and inhalation of small concentrations can cause pulmonary edema, and cases of death have been reported.
Handling osmium tetroxide with care, and disposing it appropriately is of great importance.
Fluorine: F2
Playing with fluorine gas is something most chemists are scared of.
However, due to its very particular properties, introducing fluorine atoms into molecules is of great interest in organic synthesis and all its applications.
That’s why there are many research groups specialized in doing fluorine chemistry.
But a great deal of care must be taken. The following video illustrates how fluorine can behave.
Fluorine reacts with moisture to give hydrogen fluoride, which is scary enough by itself. But contrary to HF that can be handled in solution, F2 has to be handled as a gas, and always using containers and tubing made of resistant plastic materials.
The Most Dangerous Chemicals in “Real Life”
Carbon monoxide: CO
Carbon monoxide can be a silent killer.
It can bind to the iron atom in hemoglobin, displacing oxygen, basically shutting down cell respiration.
Carbon monoxide, along with CO2, is one of the products of burning organic matter. If the concentration of oxygen is low during combustion, the amount of CO that is produced increases. This can happen in a closed fireplace inside a house, and you could get poisoned without noticing.
In fact, dozens of deaths are reported every year due to CO poisoning.
Amatoxin
We are back to natural poisons, this time discussing amatoxin.
This is a general name for several toxins with a similar structure (several amino acids arranged in a macrocyclic fashion) that are responsible for the toxicity of different mushrooms, such as the death cap (amanita phalloides).
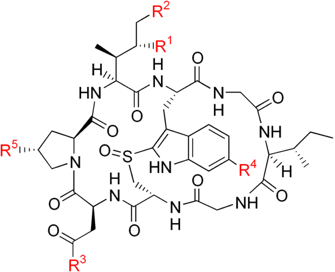
There is nothing as picking up your own shrooms out in the goods and making a great meal out of it… But it can also be dangerous!

Always make sure you known perfectly well what you are getting your hands into, and if you are in doubt, ask an expert before eating any mushrooms.
The Most Dangerous Chemicals… For Other Reasons
Azidoazide azide
This compound with a very illustrative name (and structure), is one of the most explosive chemicals that has ever been prepared.
Azidoazide azide is among the “high-nitrogen energetic materials”, and its molecular formula of C2N14 speaks for itself.
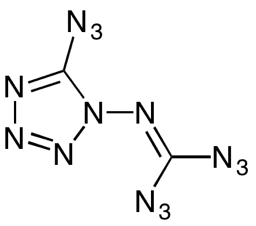
The thermodynamic feasibility of this substance to react releasing nitrogen is just HUGE.
Anything can set if off. Hit it with something, it explodes. Heat it up, it explodes.
I don’t recommend any of you ever getting close to this stuff. Instead, just check out what other people have already tested:
Thioacetone
Smell may seem like a “minor” danger sign for a chemical. But it can get beyond unpleasant, to the realm of actually being literally unbearable.
One of the most extremely unpleasant odors in chemistry comes from thioacetone.
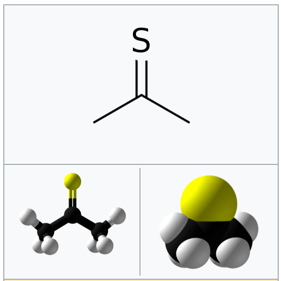
Imagine an acetone molecule in which you swap the oxygen for a sulfur atom. The result is a substance with one of the worst smells known to man.
A famous incident involving this molecule involved a couple of milliliters dropped in a German laboratory in 1889.
The result was people unconscious and vomiting in a radius of almost one kilometer (half a mile).
For its extremely foul odor, thioacetone is considered one of the most dangerous chemicals in the world.
We are now wrapping up this list of really dangerous chemicals. These guys are the actual chemicals that should scare you, but do know that everything out there is made of chemicals, which simply can be very good or very bad!
Make sure to share if you found this useful or interesting, and up next, check out our explanations for 100 chemistry facts!

Ok guess I’m not picking mushrooms or working in a laboratory ever.
I DONT WANT TO DIE.
Haha, both can be done very safely, if you are properly trained and you know what you’re doing 🙂
FOOF is such a funny name for a chemical, it makes me want to touch it. That would be a pretty bad idea though.