What is the Swern Oxidation? What can you do with this reaction? What is the mechanism of the Swern oxidation? How do you actually run this reaction in the lab and what are their most relevant practical features? These are some of the questions that I will try to answer in this guide, as someone who has run this reaction in the lab countless times.
What Is the Swern Oxidation?
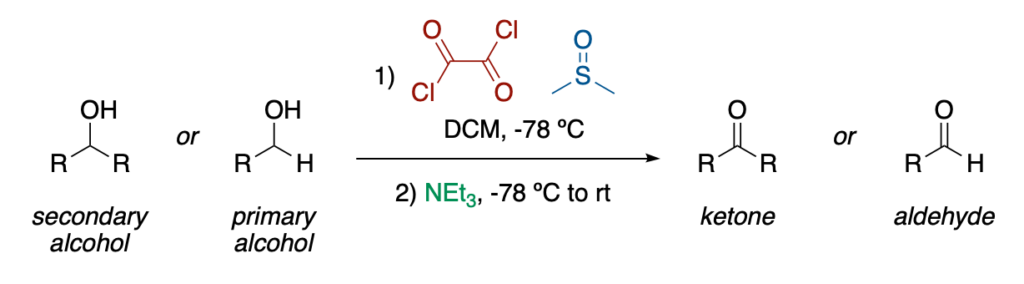
The Swern oxidation is the oxidation of a primary or secondary alcohol to an aldehyde or a ketone, respectively, by the combination of oxalyl chloride and dimethylsulfoxide followed by triethylamine.
Discovery and Applications
The Swern oxidation was first discovered by Daniel Swern and Kanji Omura in 1978. From this point, this methodology evolved into one of the most used strategies to oxidize both secondary and primary alcohols to ketones or aldehydes, respectively.
In this reaction, dimethylsulfoxide (DMSO) acts as the effective oxidizing agent, getting reduced to dimethylsulfide (DMS) as a consequence. However, DMSO by itself is not reactive enough to take part in this redox process, it needs to be activated by oxalyl chloride, (CO)₂Cl₂. This results in the formation of an adduct that can evolve into the corresponding ketone or aldehyde by action of a base (generally triethylamine), upon release of CO, CO₂, and dimethylsulfide (SMe₂), through a beautiful mechanism that is a must know for any student of organic chemistry.
This reaction has distinctive features that make it extremely popular among synthetic chemists.
Advantages and Drawbacks
One of the best features of this oxidation method is that it does not further oxidizes aldehydes to carboxylic acids, so a single 2-electron oxidation of primary alcohols can be achieved. This is often not the case with, for instance, metal-based oxidations, such as the use of potassium permanganate. Other alternatives that stop at the aldehydes, such as DMP, are usually much more expensive than the simple reagents required for the Swern.
This reaction often proceeds smoothly at very low temperatures. The usual procedure is run at -78 ºC, which means that the reaction conditions are extremely mild, this usually leads to very selective procedures that usually don’t harm other functional groups of complex molecules. On the other hand, this can also be considered a small inconvenience, since it requires setting up an acetone-dry ice bath (-78 ºC) or the use of a cryocooler instrument.
There are not many disadvantages for this reaction, as evidenced by how it has withstood the test of time, but the more characteristic one is on of the side products: dimethylsulfide is a nasty smelly gas! Make sure to run the reaction in a well-ventilated fumehood.
The Mechanism of the Swern Oxidation
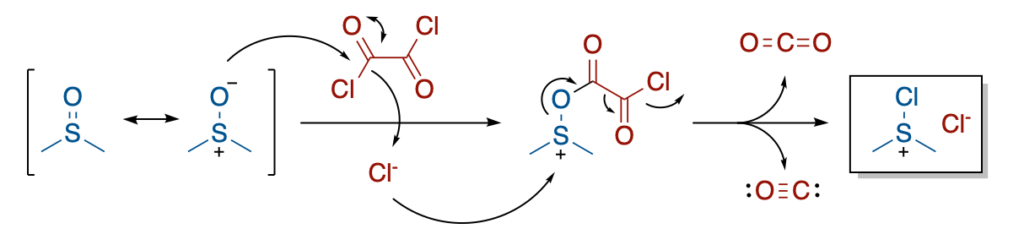
The mechanism of this oxidation starts by the activation of the oxidant (DMSO) by oxalyl chloride. This generates an adduct upon release of a chloride anion. This chloride anion acts then as nucleophile towards the electrophilic sulfur atom, which makes the intermediate collapse. This results in the release of a molecule of CO₂ and a molecule of CO. This results in the formation of Me₂Cl₂S, and highly activated oxidizing agent.
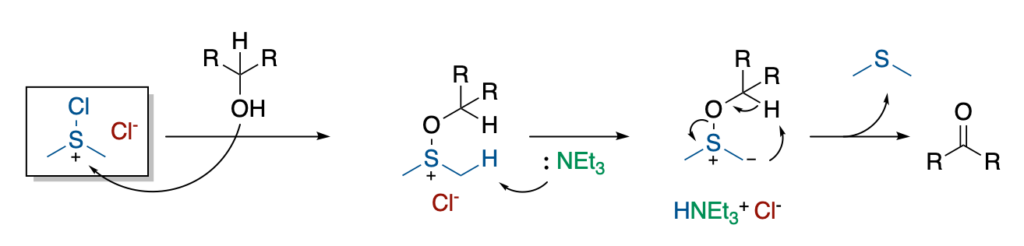
This Me₂Cl₂S intermediate can react with primary and secondary alcohols to give the adduct shown below. Then, this adduct can be deprotonated by an organic base (triethylamine) to give a sulfur ylide, upon release of triethylammonium chloride.
Finally, this ylide intermediate evolves through a 5-membered cyclic transition state to release dimethylsulfide (DMS) and the resulting oxidized product (an aldehyde or ketone).
How Do You Run a Swern Oxidation in the Lab?
As someone who has run this oxidation at work many times myself, here is a general illustration of the practical procedure for this reaction.
How to Run a Swern Oxidation
- Preparation
A flask with a stirring bar is charged with dimethylsulfoxide (3 equiv), and it is dissolved in dichloromethane (ca. 0.1–0.5 M), and the solution is cooled down to -78 ºC.
- Activation of DMSO
To the cooled solution is added oxalyl chloride (2 equiv) dropwise with a syringe. The mixture is further stirred for 30 min also at -78 ºC.
- Addition of the alcohol
After that time, the corresponding alcohol is added to the mixture as a solution in dichloromethane (if it is a solid) or neat (if it is a liquid). The resulting mixture is stirred for 1 h at -78 ºC.
- Addition of base
Then, triethylamine (4 equiv) is added to the mixture, and the mixture stirred first 10 min at -78 ºC, before removing the cooling bath. Then, thee resulting mixture is stirred at room temperature for 1 h.
- Work-up and purification
The reaction mixture is diluted with water, and extracted three times with dichloromethane. The combined organic fractions are washed first with water, then with saturated aqueous NaCl, and finally dried over anhydrous magnesium sulfate. After filtration, the solvent is removed in vacuum.
Finally you can further purify the product if it is required by flash column chromatography, and you are all done1

Really nice, much more detailed than the average SI prep 🙂
Thanks! Glad it helps!
I search for the first time site it is very nice. I like chemistry hall and your useful information.
Thanks for the kind words!
hello , i m going to start my PhD in synthesis (particularly drug) , pls write some article on how to start this , how to search for paper , literature and how to start this . how to start this phd journey .
There is one big inconvenience that I have encountered doing Swern oxidation – your DMSO needs to be really anhydrous. Seems evident, but considering how fast DMSO catches water if not under septa/inert gas, worth mentioning. In presence of water reaction does not work at all.