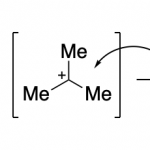
What is the Swern Oxidation? What can you do with this reaction? What is the mechanism of the Swern oxidation? How do you actually run this reaction in the lab and what are their most relevant practical features? These are some of the questions that I will try to answer in this guide, as someone who has run this reaction in the lab countless times.
What Is the Swern Oxidation?
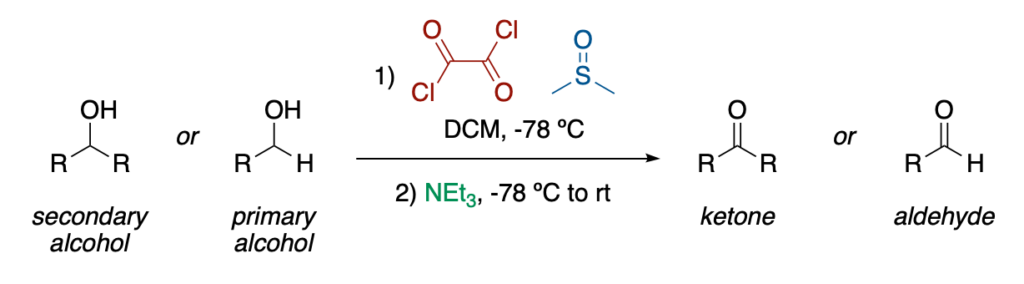
The Swern oxidation is the oxidation of a primary or secondary alcohol to an aldehyde or a ketone, respectively, by the combination of oxalyl chloride and dimethylsulfoxide followed by triethylamine.
Discovery and Applications
The Swern oxidation was first discovered by Daniel Swern and Kanji Omura in 1978. From this point, this methodology evolved into one of the most used strategies to oxidize both secondary and primary alcohols to ketones or aldehydes, respectively.
In this reaction, dimethylsulfoxide (DMSO) acts as the effective oxidizing agent, getting reduced to dimethylsulfide (DMS) as a consequence. However, DMSO by itself is not reactive enough to take part in this redox process, it needs to be activated by oxalyl chloride, (CO)₂Cl₂. This results in the formation of an adduct that can evolve into the corresponding ketone or aldehyde by action of a base (generally triethylamine), upon release of CO, CO₂, and dimethylsulfide (SMe₂), through a beautiful mechanism that is a must know for any student of organic chemistry.
This reaction has distinctive features that make it extremely popular among synthetic chemists.
Advantages and Drawbacks
One of the best features of this oxidation method is that it does not further oxidizes aldehydes to carboxylic acids, so a single 2-electron oxidation of primary alcohols can be achieved. This is often not the case with, for instance, metal-based oxidations, such as the use of potassium permanganate. Other alternatives that stop at the aldehydes, such as DMP, are usually much more expensive than the simple reagents required for the Swern.
… [Read More]