An important concern within any chemistry laboratory is the handling and storage of chemical substances regardless of the physical state in which they are. We are going to help you identify the most common (or not so common) types of chemistry flasks out there!
Through chemistry history, different materials have been employed to build these containers, although it is generally acknowledged that glass is the material of choice for most applications. From simple test tubes to the more complex micro-Kjeldahl distillation units, glass is used in most, if not all for some fields, chemical experiments performed in a laboratory.
Whether you are an experienced researcher or a curious student trying to unveil the fascinating world of chemistry, I am sure you will find in this article several interesting details that you could have missed and could be very useful once you are in front of you laboratory bench. Remember, small details make big differences!, particularly in experimental Chemistry.
Considering this, in the following paragraphs, you will find a description and useful information about the most common laboratory glassware found in any laboratory. All of them come with pictures so you can esily identify those weird pieces of glassware sitting around in the lab.
Enjoy!
• Erlenmeyer flask: It has a cone shape and a cylindrical neck, being also flat by the base. It serves to contain substances or heat them, although the shape of this flask also helps to prevent liquid spillage and facilitates swirling motion to perform titrations, or other procedures. The narrow opening of this flask also prevents dust contamination and minimizes losses by evaporation.

• Volumetric flask: A flat bottom glass container with an elongated and narrow neck that presents a line that exactly defines the volume of any liquid substance. It is generally employed to prepare solutions.

• Beaker: A cylindrical container with a flat bottom and a wide opening. It consists of presents graduations that can often be used as a measurement reference. It is commonly used to contain substances as well as to heat them.

• Measuring cylinder: It is a cylindrical and graduated glass tube that is employed to measure precisely the volume of liquid substances.

• Test Tube: These are a small cylindrical glass tube with one end open and the other closed and rounded. It is used to prepare small reactions or tests in it. They are also commonly used to collect fractions in column chromatography.

• Büchner flask: Volumetrically graduated glass container. It has a small side tube coming out of the neck which can be connected to other equipment, generally a vacuum pump. Widely employed to perform vacuum filtrations along with a Büchner funnel.

• Round-Bottom Flask: This is probably one of the most common types of chemistry flasks. Ball-like container with a wide base and narrow neck that has a stopper. It is used when the substances contained must be stirred, avoiding spillage and evaporation of gases. It can possess one, two, or three necks. They are the bread and butter for setting up chemical reactions.

• Burette: Graduated container, usually made of glass. It is a long tube of small diameter with a stopcock that allows the liquid to drip. It is used to transfer exact amounts of liquids. The most common application of this are titrations.
• Desiccator: Not really a reaction container, but you do store chemicals in it. It is a glass container with a lid that allows a tight seal. It is used to remove moisture from solid substances. Silica gel (desiccant) is placed at the bottom, while the substance to be dried is placed on a plate a few centimeters above.

• Crystallizer: A low container with a flat base. It is used in the laboratory to crystallize the solute from a solution by evaporating the solvent.

• Fleaker flask: Sometimes used to heat liquids, not a very common piece of material. It resembles an Erlenmeyer flask and a beaker. Its body is cylindrical and culminates in a neck that curves before opening into a rounded opening.

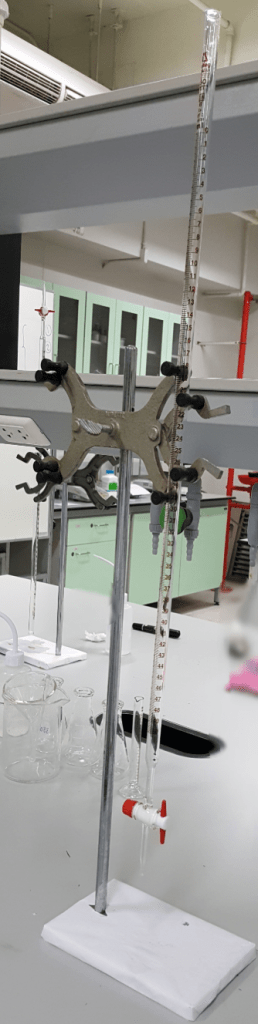
• Two-necked flasks. These are round bottom flasks with multiple (2-3) necks or entrances. One is usually employed to take chemicals in or out for the reaction. The others can have multiple uses. They can be connected to a condenser to perform reactions under reflux conditions. You can attach a dropping funnel. You can also attach a connection with a source of an inert gas to work in a closed system under argon or nitrogen, for air-sensitive reactions.
• Kohlrausch volumetric flask: They are used for sugar determination, according to the Kohlrausch method.

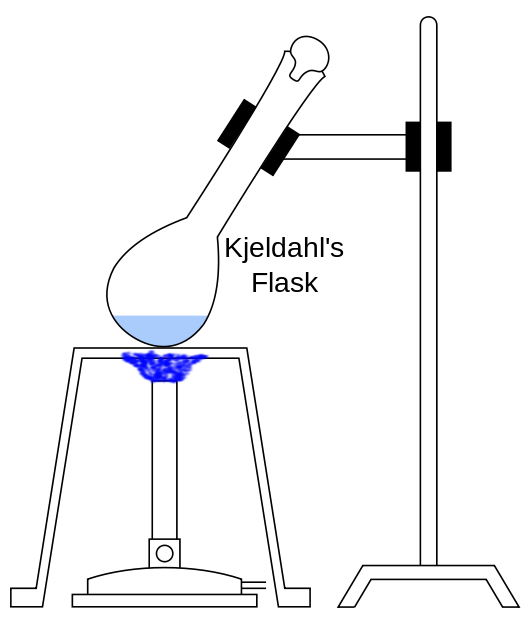
• Kjeldahl flask: It is used for the determination of organic nitrogen. Guess how: the Kjedahl method.
• Iodine flask: It is used to make iodine determinations in quantitative analysis of substances by electron exchange (oxidization-reduction) titrations that involve the use of iodine (or any other volatile chemical, for that matter). It’s quite similar to an Erlenmeyer flask (but significantly more expensive!), but is equipped with a stopper joint in order to avoid partial losses of iodine through evaporation, which would lead to errors on the quantifications.

• Saybolt flask: Used for viscosity determination.

• Fernbach flask: It is a narrow neck flask. Its shape provides a large cultivation area suitable for growing microorganisms, in liquid nutrient media. It allows faster growth, due to better ventilation.
• Mojonnier flask: It is used in fat determination, which is extracted with a mixture of ethyl ether and petroleum ether in a Mojonnier flask, the extracted fat is placed at a constant weight and expressed as a percentage of fat by weight.

• Le Chatelier flask: It is used to determine the density of things. Generally applied to determining density of stuff such as hydraulic cement, granulated blast furnace slag and fly ash for concrete, filler aggregates, and lime.

• Schlenk flask: The corner stone of working under strictly anhydrous conditions. This flask is a reaction vessel designed to perform chemical reactions which are sensitive to air. There are many variations for this, but usually it has two different necks or connections, one designed to put in the chemical reagents, and another one that is simply a connection to a Schlenk line, or source of an inert gas such as argon or nitrogen.

• Straus flask: They differ mainly from other Schlenk flasks by their neck structure. Two necks emerge from a round bottom flask, one larger than the other. The largest neck ends in a frosted glass joint and is permanently distributed by the blown glass with direct access to the flask. The smaller neck includes the thread required for a Teflon cap to be screwed perpendicular to the flask. The two necks are joined through a glass tube. The frosted glass gasket can be connected to a manifold directly or through an adapter and a hose. A typical use for these is storing anhydrous solvents with molecular sieves.

• Collector or Receiver Flask: It is a glass jar, with a very short neck, spherical body, and frosted mouth. It is designed as a piece of glass in rotary evaporators, to collect distillations of reactions with reflux. It is usually made of borosilicate glass.
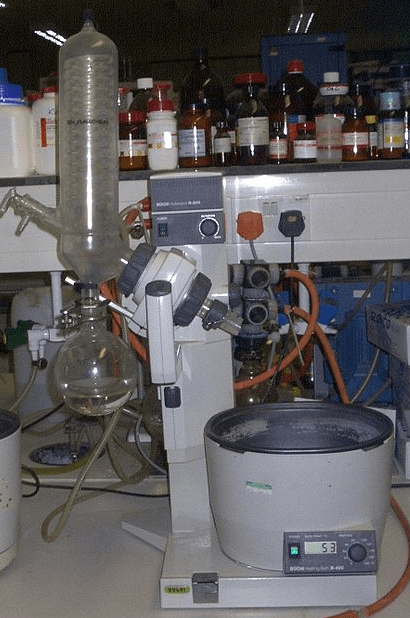
• Florentine Flask: It is a glass flask, with a long neck and spherical body. It is designed for uniform heating and is produced with different thicknesses of glass for different uses. It is usually made of borosilicate glass.

• Pear-shaped flask: It is designed for uniform heating and is produced with different thicknesses of glass for different uses. It is usually made of glass.

The biggest advantage of classic round bottomed flasks, is that its rounded base makes it easy to stir or remove its contents without being able to spill any substance out of its container as a precaution.
Pear-shaped flasks are used for evaporating solutions to dryness post-synthesis using a rotary evaporator, the ’rounded V’ shape of the flasks enables solid materials to be scraped out more efficiently than from a round-bottomed flask. Also, collecting liquids using a syringe, it’s easier with the pear-shape!
• Laboratory bottles: Made of borosilicate glass, they can withstand high temperatures and are of high chemical resistance. They are used basically to store chemicals and solutions, such as brine or ammonium chloride solutions for aqueous reaction work-ups.

• Dropper bottles with pipette: Contains substances. It has a dropper and for that reason, it allows dosing substances, such as organic solvents, in small quantities.

• Winkler oxygen bottles: It is made of clear glass, has a frosted cap and the exact volume is engraved on the bottle. It is used for the determination of dissolvable oxygen in the water.

• Big reaction vessels resistant to high temperatures or pressures. These reactors usually consist of two parts: a cylinder where the reaction mixture has to be introduced and a cap or head where there are usually different valves or connections necessary to carry out the reaction, to be able to control or monitor safety elements. In some cases, it has a heating jacket that plays the role of keeping the fluid at a constant temperature, either high or low.

• Microwave vials: Reaction vials that can be sealed with a cap, snd can resist high pressures. They are used to heat up reactions at temperatures higher than the boiling point of the employed solvent. This happens usually when heating using a microwave reactor.

• HPLC vials: These vial have a cap with a septum that can be pierced by needles, such as the ones from an HPLC or GCMS autosampler, so they are used to inject samples on instruments such as those. You can also set up small-scale chemical reactions on those if you have a stirring bar small enough!

As you can see, the list is long, and there is virtually a flask for every task you can possibly imagine. By the way, thanks to wikimedia for some of the pictures here.
Of course, you don’t really need everything if you want to set up of own home chemistry lab, but it is always good to know about them all!

The Mojonnier flask picture is labeled as a Fernbach flask
That’s true, wonder if it will get corrected soon.
Good catch guys! Corrected 🙂