You probably agree with me on the first fact: chemistry is fascinating. There are plenty of unanswered mysteries within this science, but there are also many interesting and fun chemistry facts which have been explained.
The so called central science offers us so many awesome things to think about. From chemistry facts in daily life to recent scientific discoveries. Therefore, it is not an easy task to put together an objective list of the most interesting questions and facts that you can find out there.
But we did a great deal of research to finally come up with a massive list of facts about chemistry and explanations. It has been cooking slowly for weeks, but it is finally here!
We did our best to collect no less than 100 of the most popular fun chemistry facts!

We consulted chemistry forums, and typical questions that people ask on search engines. Of course, we also used our own personal experience on what kind of chemistry questions we keep getting asked as chemists.
This is a long list of chemistry facts, so feel free to use the table of contents to navigate through it. Start reading about whatever catches you attention, and come back later to the list!
If something strikes you as an interesting fact about chemistry, we have succeeded in our mission!
We like a lot the result, and we want this to serve as a scientific outreach resource, so if you like it too, make sure to link or share this website among your students, friends, colleagues or any potential chemistry enthusiast!
By the wat, if you are interested in learning more fun chemistry with your kids, get your hands into one chemistry set now, and start enjoying the best part of chemistry: experiments!
Table of Contents
You can use the next table of contents to navigate directly to the question you are interested on the most:
What Kind of Chemistry Facts Are We Going to Learn About in This Article?
This research resulted on a selection of one hundred interesting and fun chemistry facts that can be explained and enjoyed by most audiences (don’t worry, you don’t need a PhD in chemistry to understand the explanations!).
We present them in the form of questions and also try to explain them in the more concise, and clear way as possible, for a broad audience, but without compromising scientific rigor.
We also cite reliable sources so you can expand further in all the covered topics. This list is obviously not meant to offer deep or exhaustive explanations, just the tip of the iceberg. Enough to light up you attention towards any interesting chemistry fact or story.
You will find also many facts that can be even interesting for young students or kids. If you are taking care of those, maybe you want to add some more fun to their scientific education and play with a chemistry set with them!
We have previously looked into some essential basic chemistry concepts, we suggest you to take a look at them if you are not very familiar with chemistry. Furthermore, if you are an early chemistry student, maybe you want some help to prepare for your ap chem exam. But for now let’s just get into some really interesting and fun chemistry facts.
Without further ado, let’s dive into answering the best chemistry questions that we could come up with!
We hope you enjoy the chemistry and we guarantee that you will learn something cool out of it!
1. How Do Glow Sticks Glow?
A glow stick is a self-contained light source. It is basically a plastic tube, in which different substances are contained: mainly a basic catalyst and a dye. Inside the plastic tube, there is a glass vial is filled with the other required component: hydrogen peroxide (H2O2).
When the glass vial within the plastic stick tube is broken by the user, all the components are mixed together. Then, a series of chemical reactions take place. This results in the excitation of the “dye” molecule, which upon relaxation releases light through a process known as chemiluminescence.
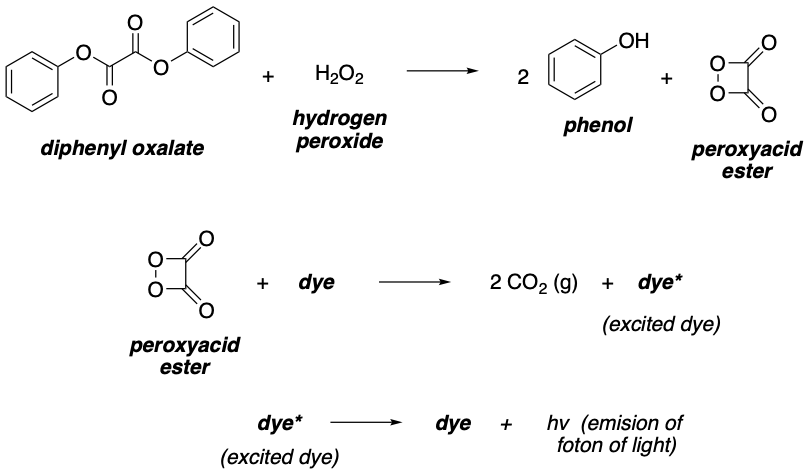
Hydrogen peroxide reacts with diphenyl oxalate giving peroxyacid ester. This molecule decomposes spontaneously, giving CO2 and releasing energy that can excite the dye molecules. The excited dye molecules can relax back, releasing photons of light of different colors. Depending on the nature of the dye, the color (wavelength) of the emitted light will be different.
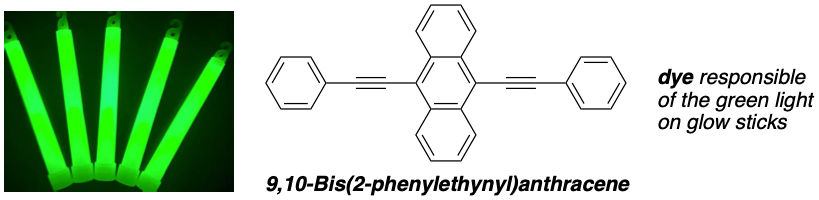
For example, typical green glow sticks use 9,10-bis(2-phenylethynyl)anthracene as dye.
2. How Do You Make Fireworks of Different Colors?
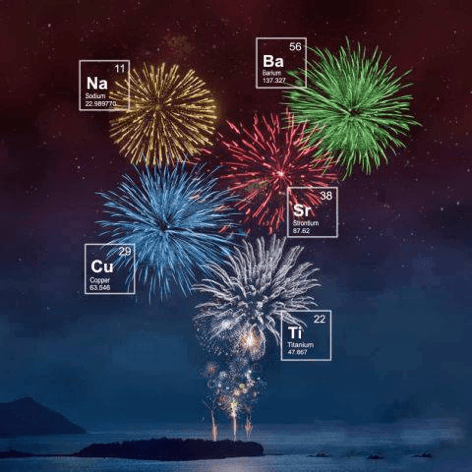
Two thousand years ago, a cook mixed three ingredients very common in any kitchen: potassium nitrate (food preservative), sulphur and charcoal. Mixed and and heated, they go off exploding on a huge bang. This is basically gunpowder.
If this mixture is put on a cane, pressure builds up giving rise to a bigger explosion. Originally, potassium nitrate was used. Potassium cations are responsible of a white color. If different salts are used instead, with different metals as cations, you get the different colors. For example, strontium salts give red colors. Iron compounds give gold-colored fireworks. Also, sodium gives yellow, barium gives green and copper gives blue. There is a great infograhic by Compound Interest about this.
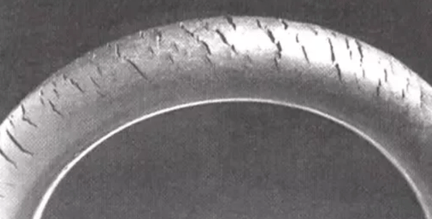
3. How Does Ozone Destroy Elastomers?
Ozone (O3) is a strongly oxidizing agent, and it is reactive towards double bonds in a chemical process known as ozonolyisis.
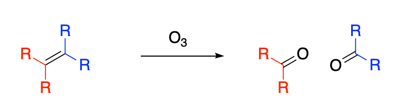
This oxidative reaction which splits double bonds into two carbonyls, is the process that disrupts the rubber polymer in elastomers, such as wheel tires and rubber tubing. But you don’t need huge amounts of ozone to get the cracking initiated, only a very small amount of O3 gets the process going.
4. Why Do We Add Fluorinated Groups to Many Drugs?
This goes beyond basic knowledge, but sometimes you see that medicinal chemists decide to “randomly” put a F atom in a molecule. This is a common pattern in drug design, which is weird because fluorine appears very rarely in naturally occurring molecules.
Fluorine is a element that is usually added to drug molecules because it can increase its selectivity. Also, adding fluorine atoms increase the solubility of the drug in fats, making it easier for it to go through body barriers. Furthermore, the simple exchange of a H atom by a F atom in a certain position, make it much more stable, and less prone to degradation by oxidation. This may have a significant positive effect in the dosage of the drug.
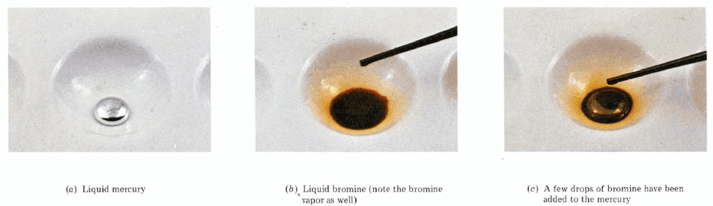
5. Which Are the Only Elements Liquid at Room Temperature?
The only pure elemental compounds (which are, compounds made of atoms of only one element) that are liquid at room temperature are Br2 (fuming orange liquid), and mercury (a metal).
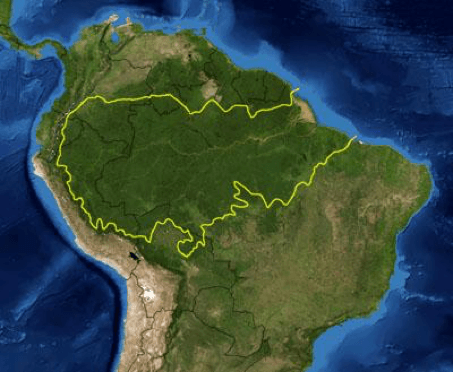
6. Where Did Most of the Oxygen in the Atmosphere Come From?
Surprisingly, 20% of the world’s oxygen is produced in the Amazon Rain forest. This is the largest rainforest on Earth. Its Basin covers 40% of the South American continent.
7. What is the Toughest Total Synthesis Ever Accomplished?
Arguably, this top spot must be given to the first total synthesis of cyanocobalamin, or vitamin B12. This vitamin is used, ironically, to treat vitamin B12 deficiency. Its first total synthesis was carried out between two huge research groups, the group of Robert B. Woodward (at Harvard University) and Albert Eschenmoser (at ETH Zurich). You can take a look at some online chemistry lectures by Woodward and other chemistry giants.
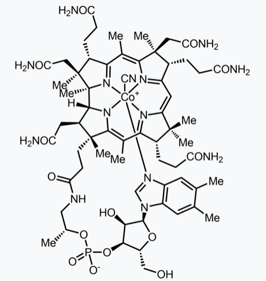
12 PhD students and 91 post-doctoral researchers were involved on this huge project, that took more than 12 years to go to completion. The total synthesis was completed in 95 steps. This step count is insanely difficult to deal with. Even if all the steps gave 90% yield, the overall yield would be (0.9^95)·100 = 0.0045%. But actually, several steps have yields lower than 20%, so the amount of starting materials required for accomplishing such task, would have been enormous. And all of this was carried out in a time in which characterization techniques such as spectroscopy were very limited to non-existent. If you are just getting into the wonders of organic chemistry, you should get your hands into one of the best textbooks out there! Also make sure to check out some organic chemistry model kits.
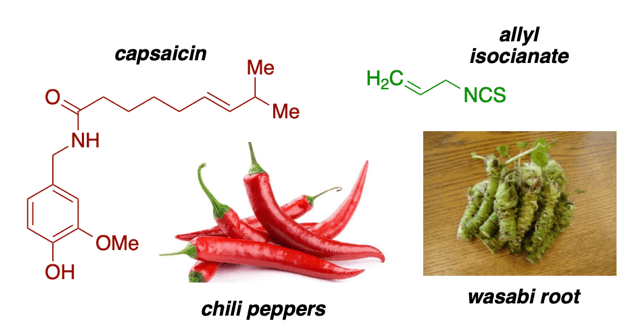
8. What Is Hot Spicy and Cold Spicy?
Peppers and similar “hot spicy” food are so due to a molecule called capsaicin. The heat of the spiciness is measured by the Scoville Scale. On the other hand, “colder” spicy condiments such as wasabi, but also mustard or horseradish, the molecule to blame is allyl isothiocyanate. The receptors for allyl isothianate can make your body sting, burn cough or choke. It gives a feeling of a less hot or “cold” spicy.
9. How Long Can Gold Wires Go?
Gold is universally used as an electricity conducting material. It is electric conductivity is not as high as copper, but it finds many uses thanks to being much more difficult to corrode (oxidize) than other metals. Besides, gold is extremely ductile. Only one ounce of metallic gold can be elongated into very thin wires of gold can be drawn into 80 km of wire! This makes the wire only five microns thick.
10. What Is a Rather Useless Property of DNA that Everyone Knows About?
A fun chemistry fact that I’ve seen circling around the internet ever since it went viral, after being published in 2013, is that apparently DNA is a flame retardant. What are the implications or the reasons behind this are still unclear, but still, something interesting to keep in mind.
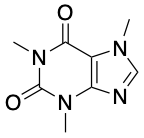
11. What’s the Difference Between Caffeine and Theine?
Caffeine was discovered first from coffee (1819) by ta German chemist, F. F. Runge. The natural function of caffeine is to act as natural defense against insects, but also is a stimulant drug that makes us feel excited, making it easier for us to wake up in the morning.
However, the term “theine” is actually a way to refer to “caffeine” when it is in tea. But the molecule is exactly the same. There are other active components in tea that have stimulant effects, such as theophylline, but they are much weaker than caffeine itself.
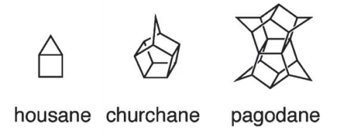
12. What are Churchane, Housane, and Padogane?
There is a great highlight published by Prof. D. Trauner in Angewandte Chemie in which he discusses “the chemist and the architect”.

Human beings are constantly getting inspiration from any conceivable source. As Trauner puts it: “To imagine a structure and then express it in material form is one of the most satisfying of human activities. It is pervasive throughout the arts and crafts and it is one of the defining features of architecture. It is also at the heart of synthetic chemistry”.
Chemists had constructed plenty of structures that resemble real constructions, and named them after them.
13. Can You Put Out a Candle with Home Made Carbon Dioxide?
Of course you can just blow a candle out. You also probably know that you can put it out by covering it with a jar or other closed container that prevents oxygen from getting in and fuelling the flame.
There is actually a cooler way! You can generate a lot of carbon dioxide gas by mixing vinegar (acetic acid) with baking soda (sodium carbonate) in a glass. The mixture will start blowing out carbon dioxide, and you can pour carefully that gas (just the gas, not the liquid!) over a candle to put it out!
14. Can Table Salt Adopt Other Stoichiometry Than NaCl (1:1)?
Yes indeed it can. Maybe you haven’t seen this in your typical intorductory inorganic chemistry textbook. However, since 2013, several salts of sodium and chlorine were predicted to be stable: Na3Cl, Na2Cl, Na2Cl2 or NaCl7. Some of these predicted structures were proved experimentally.
15. How Was the Structure of Benzene First Conceived?
Friedrich Kekulé conceived the idea of the benzene structure and its resonance forms after a dream in which he saw a snake seizing its own tail.
16. Can We Make Anthropomorphic Molecules?
Yes, many anthropomorphic (human-shaped) molecules have been made for fun by a team at Rice University, and they published in the Journal of Organic Chemistry.
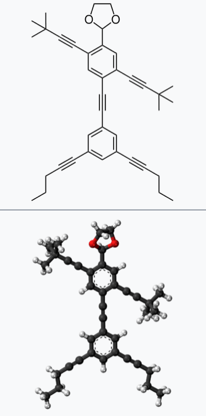
The Nanoputians are molecules that resemble human beings, and were synthesized and diversified (to give different kinds of models, from scholar to a baker)
17. What Makes Up >99% of the Normal Matter in the Universe?
Helium and hydrogen make up most of the universe. Both of them account for 98% of all matter, being roughly 73% hydrogen, and 25% helium. All the other elements make up the remaining 2% of matter. The next in the list is oxygen, making up for a tiny 0.05%. Other atomic components in this order of magnitude are neon, nitrogen, carbon and silicon. We explore this with more detail here.
18. How Was Californium Made?
Californium was made in Berkeley, by the use of a 1.5 m-diameter cyclotron. As many other artificial elements, it was made by shooting He nuclei at curium-242 nuclei. This gives up to californium-245, an isotope with a half-life time of 44 minutes.
19. What’s Especial About Vanadium Oxide?
Vanadium oxide is a weird material which is a conductor of electricity but it is not a heat conductor. This was something completely unprecedented in the world of physics until it was discovered.
20. What Are Olympic Gold Medals Made Of?
They Olympic gold medals are not completely made of gold. In fact, they are made of at least 95% of silver, containing a minimum of 6 g of gold.

Gold is much more expensive than silver. However, thanks to this “tricky” alloy, a golden medal is just worth about $550, while silver medal is around $300.
Gold is around 100 times more expensive than silver, so a full-gold Olympic medal would cost $30.000! That’s why they only add enough amount of gold to give the medal the characteristic golden color.
A bronze medal, made of cheap copper and zinc, is actually worth only $2.
21. Do Chemists Know How to Make Drugs?
This is actually one of the most typical questions chemists get asked whenever they disclose what they do. Especially after the release of certain TV show…

And the short answer is clear: Yes, they do. Easily, in many cases.
That being said, it depends on the degree of experience and on the field in which you work on. Any undergraduate, working in any field, could probably follow experimental preps or “recipes” to make a common biologically active compound.
In case of somebody working on synthetic organic chemistry, with a MSc or PhD in that field, they can probably look up how to efficiently make any drug or derivative out there, with enough resources provided and access to a scientific database. This is obviously not limited to recreational drugs, but also to most small-molecule drugs that you take when you are sick.
22. What is an Alloy?
Alloys are basically combinations of two or more different metals, or metals with non-metals. Alloys are generally produced to obtain metallic materials with a given set of desired properties.
One of the most typical alloys out there is steel. Steel is basically a combination between iron (metal) and carbon (non-metal), which present very attractive properties.
Another example is the mixture of gold and silver used in Olympic golden medals. The resulting alloy is much cheaper than pure gold, but keeping the desired golden color for the medal.
23. How do Matches Work?
Match-heads are made of a combination of chemicals. The main ingredients are potassium chlorate, sulfur and glass. No phosphorus in the match head. Red phosphorus is basically what makes up the striking surface, along with more powdered glass or sand.
The main goal of the sand/glass present in both the match head and the striking surface is to cause heat through friction.
This amount of heat promotes the transformation of red phosphorus into white phosphorus. White phosphorus is incredibly pyrophoric. It can ignite spontaneously in the presence of oxygen from air, or from potassium chlorate itself. Sulfur (along with oxygen) keeps the flame burning. The wooden stick of the match does the rest.
Make sure to check out this infographic by Compound Interest:

24. How Does the Coke+Mentos Experiment Work?
This experiment went viral a couple of years ago. Adding “Mentos” to a bottle of Coke causes a large amount of pressure to build up. This basically makes the coke go flying as a soda geyser. You probably have heard of it:
But how does it work?
Why this happens has more of a physical explanation than a chemical one. The responsible process is called “nucleation”.
Coke, or soda, is filled with carbon dioxide (“fizz”). This contained CO2 is dissolved into the liquid, and it wants out (it is a thermodynamically favorable process).
In the absence of Mentos (o whatever nucleation source you might use), this process goes on slowly. That’s why if you put the coke into a glass (which doesn’t have a lot of nucleation points, since it has an even surface), it doesn’t release a lot of CO2 at once.
Your own mouth and tongue have a fair amount of nucleation sites: irregular spots where CO2 bubbles can be easily released from the solution, that’s how you get the “fizzy” taste when you drink soda.
The surface of a Mentos is build up from a lot of microscopic layers of sugar, making it extremely irregular, full of crannies and nooks, which make up the perfect “nucleation weapon”. In contact with soda, this extremely irregular surface will make a lot of bubbles rapidly form, building up a huge pressure that results in the well known geyser!
By the way, we have covered how exactly to perform this science experiment here.
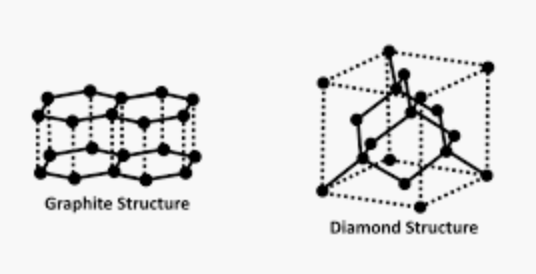
25. Why Are Graphite Rods Used in Nuclear Reactors?
Graphite is used in nuclear reactors as a moderator. Basically, a nuclear moderator decreases the speed of neutron release, allowing to control the nuclear chain reaction.
Carbon atoms in graphite can absorb the high kinetic energy that neutrons have when emitted in a fission process.
Nuclear fission reactors are based on the production of neutrons via fission processes.
Why would we want to slow down the release of neutrons? We want the neutrons to be captured by active nuclei such as uranium-235. For this to happen efficiently, without a nuclear moderator, we need to use enriched uranium (>3–5% of U-235). With a moderator, we can use natural or un-enriched uranium(LINK), much easier to access.
An alternative nuclear moderator is D2O (heavy water), but graphite rods are usually preferred, since they are solid, cheap and occupies less volume.
26. Why Do Onions Make You Cry?
A relatively complex process takes place when you cut an onion. This results on the release of propanethial-S-oxide, which is an irritant of the lachrymal glands, which release tears.
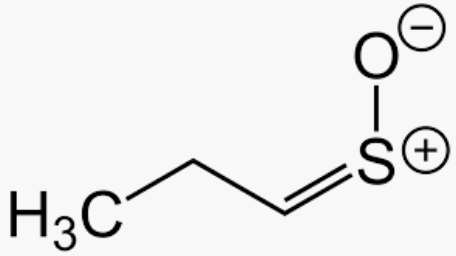
In 2002 (Imani et al), it was reported that, upon cutting, onions release an enzyme called lachrymatory-factor synthase. This enzyme transforms sulfoxides present in the onion into sulfenic acid.
Sulfenic acid gets spontaneously rearranged into propanethial-S-oxide, which through the air, goes into your eye and irritates your lachrymal glands.
27. Why Does Ice Float on Water?
Ice floats on water because it is the least dense of the two.
As a general rule, out of two different substances or materials that do not react with each other, the less dense will float on top of the denser. The density of ice is around 10% lower than the one for water.
This property is extremely important for life. Rivers and lakes freeze from the top, so animals can still survive in the liquid water below. If ice was denser than water, it would sink, displacing water to the top, freezing as well as a result. This would result on the whole river/lake freezing, killing most forms of life living within.
28. Why Is Soap Used for Cleaning?
Soap is a mixture of amphipathic molecules, which have both a hydrophobic chain and a hydrophilic head. In water, these molecules such as fatty acid carboxylates, are arranged forming micelles.
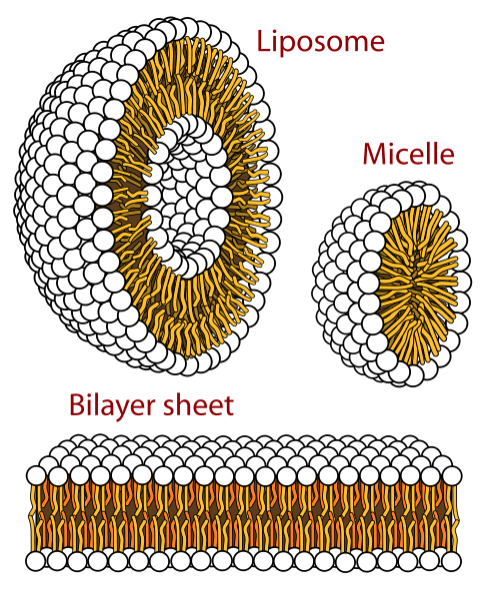
Micelles arrange the hydrophobic chains towards the center, and the hydrophilic heads towards the outside (water). Hydrophobic molecules of dirt get “trapped” in the center. This allows an easy removal of apolar compounds from clothes or your skin.
These apolar components of “dirt” would otherwise stay, since they are not soluble in water.
29. How Do You Make Soap?
As mentioned above, classical soaps are made of fatty acid carboxylates. These are typically obtained from “saponification” of fatty acids with sodium hydroxide (NaOH), also known as lye.
One source of fatty acids is virgin olive oil, or coconut oil. Heating those up to around 100 ºC, and adding a water solution of NaOH gives the corresponding mixture of sodium carboxylates. Then, fragrances are usually added before the soap mixtures are poured into a mold and slowly cooled down to room temperature.
30. What Happens to Food While Cooking it?
If you have heard that cooking is chemistry, it is totally correct. Cooking is basically bringing chemical changes to food, mostly through heating.
When you cook a piece of meat, as you heat it up, proteins start to denature. As a result, you observe the typical color change, among other things. Also, collagen starts shrinking, pushing water out. This results in meat getting drier and drier the more time you cook it.
Another cool example is the use of baking soda while baking. This is basically sodium bicarbonate (NaHCO3), which releases CO2 upon heating, helping mixtures increase in volume or “rise” while baking them.
C&EN published a cool video about the chemistry behind cooking:
31. What Venom do Wasps and Bees Have?
Both wasp and bee sting venom is basically a combination of different enzymes and small molecules. The different enzymes have the goal of breaking down cells, including neurons, which is the cause of the intense pain that we feel. Smaller molecules present in the mixture enhance the effects of these enzymes, and make it last longer.
Although the effect of both venoms is similar, the enzymatic components are mostly different. That’s why some people can be allergic to one of the two but not to the other.
You can read further about this in our previous post about sting venom.
Furthermore, don’t forget to check out the list of most dangerous chemicals and poisons that we have put together on this other post!
32. Can You Turn Lead into Gold?
The short answer is yes, but not by chemical means, and the physical process is totally not worth it. Alchemy, the protoscience of chemistry, believed that it was possible to transform other metals into gold by the use of a “philosopher stone”. Now we know that is not possible, it was just myths and magic.
However, it can be actually done by using nuclear transmutation.
The difference between the two metals is the atomic number, defined by the number of protons they have in their nuclei. You can go from lead (it has 82 protons) to gold (it has 79 protons) by removing 3 protons from the nucleus. This can be straightforwardly achieved using particle accelerators. In fact, this is the way new elements are discovered, by using magnetic and electrical fields to accelerate particles, which then impact a starting nucleus. This impact can remove protons or neutrons from the nucleus, giving rise to new elements, or isotopes, respectively.
In summary, we can use physics to transform lead into gold, but the process is incredibly far from being economically viable!
33. Why Do We Put Salt Into Icy Roads?
This is due to colligative properties, specifically freezing point depression. When dissolving a salt, such as NaCl, in water, we can make its melting point go down from 0 ºC all the way to -20 ºC, or lower. This way, water will stay in liquid form even at sub-0 ºC temperatures, getting rid of ice on the roads.

34. Why Does Asparagus Make Your Pee Smell?
Asparagus have non-volatile sulfur-containing compounds. During digestion, we break down those compounds giving rise to volatile sulfur-containing chemicals, that can get to your nose through air. These compounds are smelly only to around 25% of the population: not all of us have the gene that allows smelling those compounds.

So, everyone produces those smelly volatile chemicals, but only a fraction of the population can actually detect them. we have posted also an entire account on this phenomenon, check out more about the chemistry behind asparagus!
35. Why Does the Planet Uranus, Rich in Methane and Hydrogen, not Burn?
Planet Uranus is indeed rich in extremely flammable gases, methane and hydrogen. But the burning of these gases requires oxygen. While we take oxygen for granted in Earth, Uranus simply doesn’t have enough for the flammable gases to burn.
36. How Does the Sun Burn without Oxygen?
Sun is made mostly of hydrogen (besides helium), which is a highly flammable gas. But, as in the case of planet Uranus, there is no oxygen at the Sun.

In classical terms, we need oxygen for a fire to burn. But the Sun is not actually on fire. Its heat and light come from nuclear fusion reactions, mainly combining hydrogen to make helium. This process does not require oxygen to happen.
37. Can You Burn a Diamond?
Diamonds are made of pure carbon, so it makes sense to think that they could burn under a oxygen atmosphere to produce carbon dioxide. But since the three dimensional arrangement of the diamond is so tight and difficult to disrupt, very high temperatures (in the order of 1000 ºC) would be required.
38. Can You Cool Pure Liquid Water Below Zero Degrees?
We have seen that adding other compounds to water, such as salts, allow us to decrease its freezing point. But what about pure water?
Yes, you can cool liquid water below zero degrees Celsius if you increase the pressure.
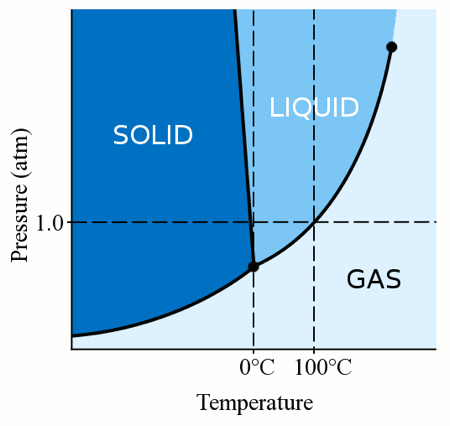
As you can see in the diagram, as soon as you go up from 1 atm of pressure, the melting point of water decreases.
39. What Is the Ozone Layer?
It is one of the layers in Earth stratosphere, around 10 km up from the ground. It has a high concentration of ozone (O3). This gas is responsible for the absorption of most of the ultraviolet (UV) radiation coming from the Sun. Without it, the cases of sunburn, skin cancer or cataracts would increase dramatically.
40. What Is a Flame?
A flame is basically the part of a fire (an exothermic oxidation, or combustion generally by oxygen) that we can see with our eyes. The temperature or the color of flames depends of what is being combusted. Flame tests are performed in extremely hot flames, in which there are ionic gaseous components. These can be considered to be plasma.
41. Why Is Water Blue?
As some suggest, water is not blue due to the reflection of the sky. Water is blue on its own. Any object shows a given color because it is absorbing visible light from some other wavelength preferentially. Water has a weak absorption band in the red part of the visible spectra (close to the infrared zone). For this reason, water shows a blue color. Since this red absorption band is so weak, we can only observe the blue color if we have a massive volume of water. That is why water in a glass appears to be colorless, but bigger volumes such as oceans, lakes, or swimming pools, are blue.
This reading in the Journal of Chemical Education is highly recommended if you want to expand further on the subject.
42. What Is the Difference Between Glass and Crystal?
Although some people may use these words interchangeably, they are not the same. In fact, both things are somehow opposite by definition. A crystal is a solid substance that presents a highly ordered arrangement of its microscopic components. On the other hand glass is an amorphous non-crystalline solid, which are usually produced by rapid cooling of melted amorphous materials, such as silica, or SiO2.
43. Can You Burn a Metal?
You definitely can burn a metal. Some examples are thermite (in which you are basically burning aluminum) or fireworks.
44. Are Two Atoms of the Same Element Exactly the Same?
Two atoms of the same elements are exactly the same if and only if they have the exact same molecular, atomic, electronic and nuclear states. This is extremely difficult to achieve, so much so that a physics Nobel prize was awarded on this regard.
For example, sodium and chloride from NaCl are the same elements than of metallic sodium or chlorine gas: their molecular/atomic state is completely different.
Another example are different isotopes of the same atom. Not all bromine atoms in NaBr are isotopically the same. Some have 79 neutrons on its nucleus and some have 81.
45. What Is the Origin of Life?
This is an extremely complex question to answer, but chemistry sure is central to this phenomenon. The origin of life is basically the transition from chemistry to biology, at it is estimated to have happened on Earth around 4000 million years ago, when gaseous water first condensed to give liquid water.
46. Is There Any Liquid Metal at Room Temperature?
Yes. The only metal liquid at room temperature is mercury. In fact, mercury stays a liquid until it is cooled down below -39 ºC. Gallium is also a fun metal. Its melting point is 30 ºC, so it stays solid at room temperature but it melts at the 37 ºC of your hands!
Do you want to know if you could stand on liquid mercury? Take a look at this video!
47. Why Do Some Balloons Float?
They float if they are filled with a gas significantly lighter than air. Helium is less dense than air. Therefore, helium balloons will float. On the other hand, air-filled balloons will not float due to the weight of the balloon’s rubber itself.
48. Why is Mars Red?
The red planet gets its name from iron oxide, or rust, Fe2O3. Mars is covered with this orange-red material. If you see the sky from Mars, it will appear light orange due to iron oxide particles suspended on its atmosphere.
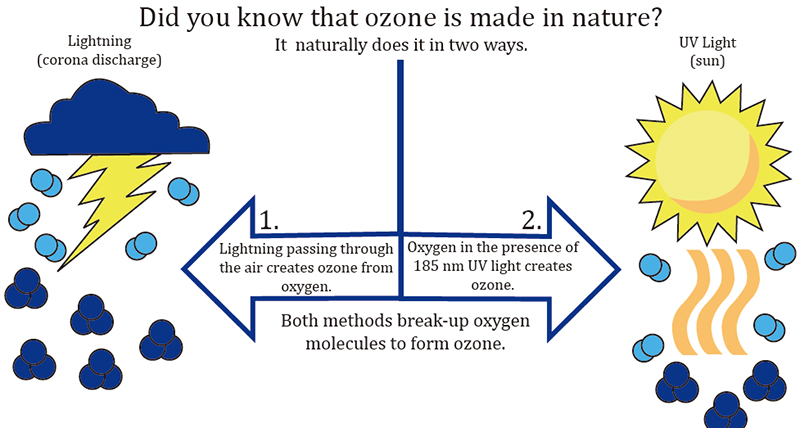
49. Why Do Storms Smell Like Ozone?
Ozone (O3) has a particular smell, which you can smell after heady rains in thunderstorms. Lightning heats up the air to the level of 50.000 degrees Celsius. This extreme conditions can cause some of the oxygen (O2) of the air to recombine into ozone, which we can smell.
Nature creates ozone through lightning and sunlight:
50. Is Glass a Solid or a Liquid?
Glass is not a liquid; it is an amorphous solid. With amorphous we mean that it has no microcrystalline order. With solid we basically mean that you can grab it without losing its shape.
51. How Old are Hydrogen Atoms in Our Body?
Hydrogen atoms, together with helium atoms, were created right after the Big Bang. This happened around 13.7 billion years ago! So the hydrogen atoms that make up your body are 13.7 billion years old.
52. Can You Freeze Air?
Yes, you can. Air is mainly a mixture of nitrogen gas (N2) and oxygen gas (O2). Their freezing points are -210 ºC and -219 ºC respectively, so below -220 ºC, air would freeze. This has been achieved by using liquid helium. Helium, however, is the only known gas that does not freeze. Helium liquefies at -270 ºC.
53. Can a Substance Solidify Upon Heating?
Apart from complex mixtures, such as certain foods, which can solidify upon heating (for example, an egg), there are examples of simpler liquids that defy the rules, and freeze upon heating. A mixture of two organic components which is liquid at room temperature, solidifies in the range from 45 to 75 ºC. The mixture takes the state of a sol-gel.
54. Can We Reach Absolute Zero Temperature?
Absolute zero (0 K or -273.15 ºC) is a theoretical minimum, in which atoms would stop moving, and cannot be achieved. However, thanks to cryocooling refrigeration techniques, we can come close, in the range of a billionth of 1 K. Surprisingly cold! But never absolute zero.
The idea of absolute zero was actually pioneered by Robert Boyle himself!
55. Why Does High Air Humidity Make it Feel Hotter?
The natural mechanism of our body to cool itself down is sweating. Evaporation of sweat from our skin takes up energy from our bodies, cooling ourselves down. The more water there is already in the air (higher humidity), the more difficult this evaporation process take place. Therefore, higher concentration of water on air, makes us feel hotter because we cannot cool down efficiently.
56. Can You Die for Drinking too Much Water?
Hyponatremia (which means “low in sodium”) is what water intoxication is called. Approximately, drinking 6 litters of water in a relatively short time, can cause serious injuries, even death! This happens because huge amounts of water make your blood concentration of sodium (or other electrolytes) drop drastically, making your cells accumulate too much water inside, swell, and even rupturing.

This would happen more easily if you drank distilled water (which is not dangerous in small amount, just as regular water).
57. Why Car Airbags Are Filled with Sodium Azide, a Very Toxic Substance?
Airbags are actually not filled with some compressed gas. Chemistry takes action when airbags inflate. They are filled with around 100 grams of sodium azide (NaN3), which upon heating (which is triggered by an igniter that goes off upon collision detection) decomposes to give N2 gas (more than 50 L, enough to fill a typical airbag) and sodium (Na) metal. Since sodium metal is potentially explosive, the airbags also contain several compounds that would react quickly with sodium, to avoid any danger.
58. How Many Molecules Are in a Rubber Tire?
In a nutshell, you could say that it is just a large single molecule, a polymer with a huge molecular weight. In reality, it is a bit of a grey area, and calling it a single molecule is misleading.
Rubber tires are actually made by combination or binding of different polymeric chains. These are combined together in the process of vulcanization in which sulfur present in these chains forms covalent bonds that attach the polymeric chains together.
This process basically links all the individual chains, forming a cross-linked network. You can say that the result is a single molecule. However, a more accurate description would be defining this kind of polymers as “molecules-of-molecules”.
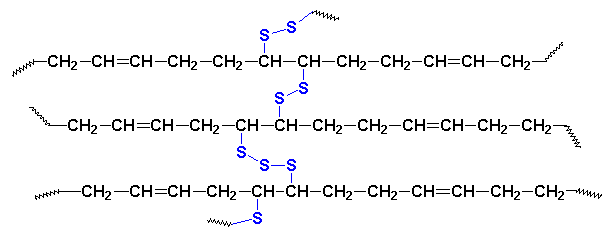
59. Why Does Water Expand when Freezing?
This is mainly due to hydrogen bonding in water. Water behaves weirdly because of this. From 4 ºC (when it reaches the maximum density) above, water behaves “normally”, expanding upon heating and contracting upon cooling. In the 0–4 ºC range, it actually contracts upon heating.
As water goes below 0 ºC, crystalline networks of ice form, in which the orientation of hydrogen bonding changes, arranging water molecules further away from each other. This results on ice being less dense than liquid water.
60. Who Discovered the Periodic Table?
The first disclosure of a periodic table as we know it today, which celebrates its 150 anniversary in 2019, was made by Dmitri Mendeleev in 1869. He published an arrangement of the known elements by the time ordered by atomic mass. This allowed, not only prediction and understanding of properties, but also to predict the discovery of empty blank spaces in his original periodic table!
61. How Does Helium Change Your Voice?
Sound travels though helium, a lighter gas, much faster than it does through air, a heavier gas. Sound travels around 2-3 times faster through helium, making high frequencies sound louder than low frequencies, making your overall voice sound higher in a funny way.
If you were to do the opposite: inhaling a gas denser than air, the effect would be your voice sounding lower for the exact same reason.
62. How Did We Discover that Diamonds Are Made of Carbon?
The discovery of the composition of diamonds is attributed to Antoine Lavoisier, at the 18th century. Lavoisier built a solar furnace, which is a tool that is used to focus sun rays. This technique allowed him to burn a diamond. Analysis of the resulting residue let us learn that the composition of the diamond was simply carbon, common coal. The work carbon comes exactly from French coal “charbon”.
63. What Would Happen if you Peed in Space?
This works for water, or any kind of aqueous solution such as pee. If you put water outside your spaceship, even though it would be dramatically below its freezing point, it would vaporize and go into gas phase right away. This is because of the lack of air pressure there is in space. It would be like submitting it to very high vacuum.
Nevertheless, eventually, it would freeze, but not before turning into a gas first!
64. What Is Dry Ice?
Dry ice is basically the name we use for solid (frozen) carbon dioxide, or CO2. It has a surface temperature of -78 ºC, and it is widely used as cryo-cooling agent.
65. What Color is Oxygen?
As a gas, elemental oxygen (O2) is colorless, odorless and tasteless. However, if you cool it down enough to liquefy or freeze it, it becomes pale blue.

This is due to oxygen becoming paramagnetic when it condenses into liquid or solid phase. The unpaired electrons originate a “magnetic asymmetry” in the molecules. This creates a absorption band in the visible spectrum (very much as why water is blue), which absorbs red light, resulting in a blue color.
In another post, we have covered the color of oxygen and its properties in more detail.
66. What Happens When You Add Table Salt to a Volume of Water?
Volumes are not always additive. When you add table salt, or NaCl to water, you are increasing the density of water. This happens due to positive interactions between water molecules and Na+ and Cl– ions.
Since the resulting mixture is denser, the total volume will decrease and be lower than the actual sum of water volume and added salt volume.
67. What Happens if You Mix Half a Liter of Alcohol and Half a Liter of Water?
This is another example of non-additive volumes. Positive interactions between water and alcohol (ethanol) molecules, make the resulting mixture occupy less than 1 L of total volume. You can think of it as if you mixed two substances which molecules can be easily held between the molecules of the other species. Imagine mixing 0.5 L of sand and 0.5 L of water. The resulting mixture will barely occupy more than a single half liter. For this case is the same, although at a much lower extent.
68. Can You Taste Food without Saliva?
Our mouth taste receptors are made to work by detecting dissolved substances. If you don’t have saliva to dissolve the molecules in food responsible for its flavor, you will not be able to taste it.
69. What Color is Lobster Blood?
Lobsters have blue blood. As you know, vertebrates and most other animals have red blood. This red color comes from hemoglobin, which is basically a protein containing an iron-porphyrin coordination complex. On the other hand, animals such as lobsters have a different protein, called hemocyanin. This protein has an active site which contains a coordinated copper atom, responsible for the blue color. This is also the case for other animals, such as snails and other mollusks.
Check out this summary about the origin of the different colors of blood!

70. Can Goldfishes See Colors?
As surprisingly as it may seem, goldfish have a very specialized vision in which they rely on for finding food. Human beings can only see three primary colors (red, yellow and blue). However, goldfish see a fourth primary color (they are tetrachromate), which is part of the ultraviolet spectra. This is also the case for the zebrafish.
Being able to see UV light allows these animals to detect very subtle movements in water, helping them find prey, such as shrimp or worms.
71. Why Do Fresh Eggs Sink, and Rotten Eggs Float?
A classical trick to know whether we can still eat an egg (if they are fresh enough) is putting them in a bowl of water. If the egg sinks, it means that it is still denser than water, which is the natural state if they are still fresh.
As decomposition takes place, solid and liquid matter is transformed into gas. Gaseous pressure builds up, and since the egg shell is porous, this gas starts escaping. This loss of mass, eventually leads to the density of the egg being lower than water’s. This makes the egg float. This represents an easy way to tell if an egg has undergone too much decomposition to be eaten (if it is rotten).
72. How Hot Does a Lightning Strike Get? Is it Hotter than the Sun?
Lightning is incredibly hot! They can reach temperatures of around 30.000 ºC, which is around 5 times the temperature of the surface of the Sun. Keep in mind that this is just its surface, the core of the Sun reaches several million degrees, which is much more than lightings.
73. Why Does Wildfire Spread Uphill Quicker than Downhill?
A fire needs a combination of fuel (the trees) and oxygen to keep going. This combination is fed more easily to the fire if it is moving uphill, when the fire from the top of one tree can start burning the bottom of the next one, which is in a place with much more available oxygen and unburnt material. Picture how matches burn: they also burn much faster if held upside down than if you leave it the correct way. The case is very similar with forest fires.
74. Do Frogs Need to Drink?
Frogs do not need to drink using their mouths. The absorb water through their skin. They have a skin-area called “drinking patch”, on their bellies, which they use to get all the water they need.
75. What is the Hardest Chemical in Your Body?
The hardest substance on the human body is enamel, the external tissue that covers teeth. It is made up almost exclusively by minerals, being calcium phosphate the main component.

76. What’s the Role of Ethylene in Fruit Ripening?
Ethylene is a gas that acts as a growth hormone for plants. It can be released by plants and fruits, and at the same time, it regulates processes such as aging or ripening. Ripening is basically the set of changes that fruit undergoes over time: generally softening, and changes of color or texture. These changes can be triggered by ethylene. An example of a fruit that produces a lot of ethylene are bananas. This is why storing other fruits near bananas, will make them ripen faster.
77. What is the Mole Day?
The mole day is a sort of funny celebration day for chemists, which takes place on October 23 between 6:02 am and 6:02 pm. This makes the date to be 6:02 10/23. This celebrates basically Avogadro constant, which is roughly 6.02·1023.
78. Do Metals Have Antibacterial Properties?
Yes, some metals (in their actual pure metallic form, not as salts or complexes) do have antibacterial properties. The most common one is copper, or alloys of copper, these metallic substances are natural antibacterial compounds. It was also found more recently that other pure metals, such as titanium, zinc, or nickel also have antibacterial properties.
79. Can We Freeze Helium?
Helium is the only known substance that cannot be frozen at atmospheric pressure. However, under pressures higher than 20 atm, liquid helium (which is usually employed to cool down to cryogenic pressures, and is able to freeze other gases), can be pushed into the solid phase. Helium melting conditions are located at 25 atm and 0.95 K, which is less than 1 degree above above absolute zero temperature!
80. Is There Helium on Earth, and How Do We Collect It?
There is a lot of helium on the universe. In fact, it is the second most abundant element next to hydrogen. However, it is not that abundant on Earth. But still, there is some, and it is located underground. It is basically extracted in the same process that we use to get natural gas from mines. The amount of helium present in natural gas is then separated using cryogenic separation processes.
81. How Much Carbon Is in the Human Body?
We can make a lot of pencils with the carbon in each of our bodies! Roughly 20% of the human body is carbon. Taking an average adult of 70 kg, that gives you around 14 kg of carbon. If that amount of organic carbon was transformed into graphite, we could make almost 10.000 pencils (which contain 1–2 grams of graphite) out of it.
82. Do Mosquitoes Bite More Girls than Boys?
A widespread idea is that mosquitoes are generally more prone to bite women than men, because estrogen can attract them. This is not really the case according to a study: the main factor playing a role here is heat emission. Mosquitos get to you following the heat that our bodies emit. Also, higher emission of carbon dioxide correlates with a mosquito wanting to bite you more, as well.
Larger people usually emit more heat and CO2, so these will usually be bitten by mosquitoes most. Men are usually larger than woman, so they would be bitten more often.
Similarly, pregnant women, who exhale more CO2 and usually show higher body temperatures, can be easily detected by mosquitoes.
83. What Is the Softest Substance?
It is common knowledge that the hardest material known on Earth are diamonds, but defining the softest is not as simple.
Softness is the tendency of a substance to get deformed (and stay deformed) when pressure is applied to it.
The classical test to evaluate hardness/softness is Mohs test, in which two materials are rubbed against each other, to see which one scratches which. According to this test, talc, a mineral made of hydrated magnesium silicate, defines the softest point in the 1–10 Mohs scale.

84. What Is the First Element Ever Created?
The first Nature-made elements were helium and hydrogen. They formed after Big Bang, within an extremely hot environment, as a result of combination of subatomic particles. Quarks combined, originating protons and neutrons, which got together giving nuclei. Then, electrons eventually combined with nuclei, creating the first hydrogen and helium atoms. As for the first human-made element, the answer would be technetium.
85. Which is the Heaviest Element in the World?
In terms of density, the heaviest element is osmium (22.59 g/cm3), followed closely by iridium (22.56 g/cm3).
In terms of highest atomic number, it is something that changes every time a new, heavier element is discovered. The natural element with the largest atomic number is uranium (with an atomic number of 92). However, many synthetic heavier elements have been discovered, being oganesson, previously known as ununoctium, (atomic number of 118) the one that holds the first place. It was first synthesized in 2002.
86. What Is the Rarest Element on Earth?
Out of all the natural elements on Earth, the fifth halogen, astatine, is the least abundant one. It is so rare that only 30 grams of astatine can be found in our entire planet!
87. How Dangerous is Hydrofluoric Acid?
Out of all of the hydrogen halides, HF or hydrofluoric acid is actually the least acidic, but it is also arguably the most dangerous. It can not only be fatal if swallowed or breathed, but also very dangerous in contact with your skin. HF can easily go through our skin, attacking and heavily damaging our tissues (including bones) from the inside.
Check out some experiments by Periodic Videos:
88. How Much Gold Is There on Earth?
The usual estimation says that there is around 170.000 metric tons of gold on Earth. This amount would fit in a cube sized around 21×21 meters.
89. Why Do Coins Have a Smell?
Coins, and metals in general, actually don’t have a smell. Our own bodies are responsible for the typical “metallic odor” that we associate with them.
Upon contact with some metals (including iron), 1-octen-3-one is formed as a result of the decomposition of oils present in our skin. This chemical is the real responsible of the smell that we associate coins or metals with.
90. Why Gold Does not Present a Silvery Shine as Most Metals?
This is not an easy question to break down in a few lines. The answer relies on quantum chemistry and relativistic effects.
Most metals have no color, in the sense that they do not absorb photons on the visible light wavelength range. They reflect all the visible light, resulting in the typical silvery shine. Further reading.
However, due to relativistic effects, some of the outer electrons of gold atoms, move much faster than usual. This quantum effect shifts the absorption range of gold so it covers some of the visible spectrum. Like so, gold can absorb some blue light while it reflects the rest of the visible light, resulting in this shinning yellow or golden color.
91. What’s Special About Gallium?
Gallium is a metal with an unusually large liquid state range, which goes from 303 degrees Kelvin (30 ºC, that’s why it melts in your hands) up to 2477 K. This is because it has a significantly anomalous crystal structure compared with most metals.
Check out how body temperature can melt gallium!
92. How Many Water Molecules Are in a Bucket?
Let’s say we have a 1 L bucket of water. One liter of water is roughly 1000 grams, which translates into 56 (1000 g/18 g/mol) moles of H2O. If we known that each mole of a compound contains around 6.022·1023 molecules of that compound (Avogadro’s constant), we will have 3.37·1025 water molecules inside the 1 L bucket. This number is around 4000 times larger than the estimated number of grains of sand on the entire Earth!
93. Does the Hole in the Ozone Layer Still Exist?
The layer of ozone present on the stratosphere protects us from most dangerous UV radiation coming from the Sun.
Over the 1980’s and 1990’s, a hole (more accurately, a zone of partial depletion or lower ozone concentration) in this layer was dangerously growing in size, as a result of people abusing the use of CFC (chlorofluorocarbons) compounds.
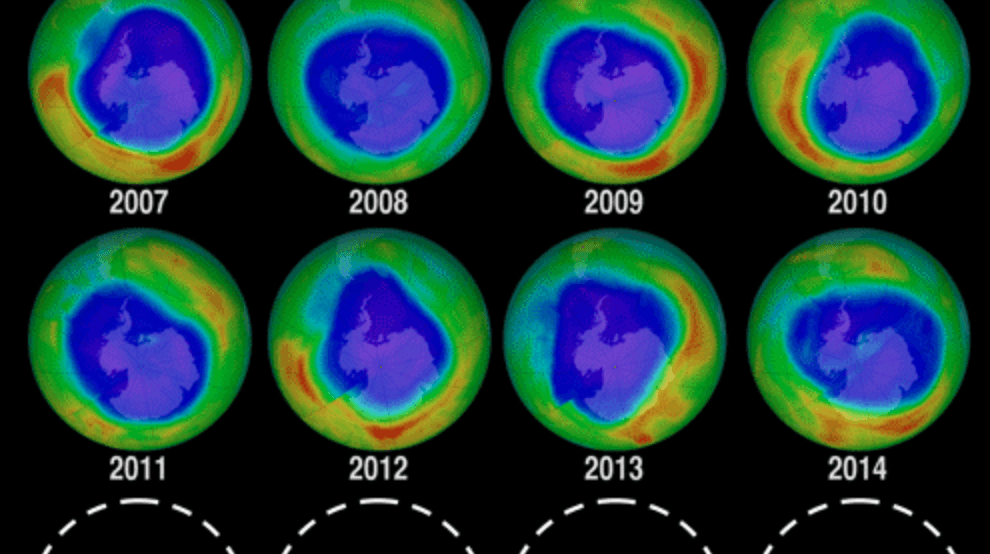
Fortunately, after prohibition of many substances that were damaging the ozone layer, the hole present over Antarctica started to shrink, going back to the size that it had before the 1980’s.
94. What Is the First Time that Chemistry Has Been Used?
Modern chemistry, together with modern science in general, it’s a relatively new thing. But human kind has been using chemical processes even by 1000 BC! Technologies such as extraction of metals from ores, medicine from plants or fermenting wine, are nothing more than chemical processes, discovered by people thousands of years back.
95. Can We See Atoms and Molecules?
Yes, thanks to techniques such as atomic force microscopy. You can consult our account on the imaging of atoms and molecules by those techniques.
96. Can Molecules Walk?
Yes! The team of Leigh and coworkers has reported molecular machines that appear to be walking at the molecular level. A video is worth a million words: If you have time and you are really interested in a subject that got awarded the Nobel Prize in 2016, take a look at this lecture by Prof. David Leigh. Watch out for the magic tricks 😉
97. How Did They Come Up with Coca-Cola?
John Pemberton, an American Civil War veteran, who was wounded during this period, dedicated the rest of his life to the development of a new medicine to use as painkiller. Most of his attempts were unsuccessful, expect for a beverage based on the coca plant, which helped calming nervousness. Pemberton sold the recipe to a businessman before he died, who turned into the drink that we all know today.
98. What is More Complex, the Universe or Chess?
As it is agreed by most physicists, the entire universe is made up of about 1080 atoms. This is a 10 followed by a lot of zeroes. A gigantic number. However, a mathematical estimation of the possible moves that could happen in a game of chess, found it to be 10120. This points to chess not being so boring as it may seem…
99. What Happens if You Clean Your Hands with Bleach?
When you make alkalis as lye react with fatty acids, you get soap. If you use alkalis, such as bleach to wash your hands, something similar is happening. You are turning the fatty acids in your hands into soap, making your hands weirdly smooth and slippery. Now you are turning your hands into soap!
100. Are We Made Mostly of Empty Space?
Human beings are made of organs, proteins, molecules, atoms. Atoms. We are made of atoms, which on themselves are actually made of almost nothing: empty space. Apart from the subatomic particles, (neutrons, protons and electrons), the volume of an atom is >99% plain empty space. Then again, these atoms are held together by different kinds of atomic forces, so, if the basic units that build us up are empty atoms… We are literally made by >99% of empty space!
I leave you with this quote from the Institute of Physics:

101. BONUS: Should I Trust an Atom?
Never! They make up everything!
(Hint: you can check a t-shirt of this fact on this list of chemistry gifts)
Hope You Have Enjoyed Our List of 100 Fun Chemistry Facts!
We have come to the end of the largest list of interesting and fun chemistry facts on the internet! I’m sure that you have learned something out of it.
Be sure to discuss in the comment section whatever you want us to look further into!
We appreciate any feedback.
If there is a particular question or topic that you would like to see expanded further, be sure to let us know and will try to tackle it in the future.
Now its your turn to interact! Make sure to share this content with everybody who could enjoy this gigantic compilation!
Our only mission with this compilation is making people interested in chemistry. Therefore, any help that you can provide for making this post reach any audience that could appreciate it, will be more than appreciated!

This was fantastic and I shared it with young high school chemistry students.
Patricia Cleary PhD
Thanks for the kind words and for sharing, I’m glad it helps!
IT IS AN EXCELLENT COLLECTION OF FACTS WHICH ARE INTERESTING. I SHARED ALL MY UNDERGRADUATE STUDENTS AND ALSO MY DAUGHTER. NOW SHE IS IN LOVE WITH CHEMISTRY. WAITING FOR ANOTHER 100 FACTS ON SERENDIPITY AND CHEMISTRY
Thanks for the kind words. This is my whole goal on writing such articles, spreading around the world how great chemistry is, I’m glad it helped!
This article is absolutely awesome! It really did increase my interest in the field of chemistry and provides me useful answers to my daily wonders! 🙂
Glad you find it useful and interesting!
Yes i love its very much THIS
This is terrific and I shared it with my daughter who is loving high school chemistry.
One correction, regarding why graphite rods are used as a moderator in a nuclear reactor. The answer stated that the graphite slows down the neutron release. This is not technically true, I don’t think. It slows the speed of the neutrons that are released, which in turn makes them more susceptible to interacting with other uranium atoms. This ultimately increases the number of neutrons released overall eventually creating a sustained reaction called “the point of criticality”. Source- former nuclear engineer
Re:#46 Mercury is the only pure element that is a liquid metal at standard temperature and pressure. But it’s not the only metal that has that property. As #22 reminds us, alloys are mixtures of metals made to have desired properties, such as being a liquid at room temperature. As compound often has a lower melting point when impurities are present, some gallium alloys would be expected to have melting points below that of pure gallium.
Alloying gallium with other metals that have low melting points such as indium and tin, produces alloys that are liquid at room temperatures. For example Galinstan, an alloy of 68% gallium, 22% indium and 10% tin by weight, melts at a cool 11°C (51.8°F). Or Indalloy-46L, which melts at 8°C (46.4°F).
Galinstan, like other liquid metals, supercools very readily and often remains liquid to temperatures as low as -19°C. This is often mistakenly given as Galinstan’s freezing point. Like mercury, these metals are liquid at room temps. But unlike mercury, they aren’t particularly toxic.